Robert B. Quast
@rbquast.bsky.social
30 followers
11 following
1 posts
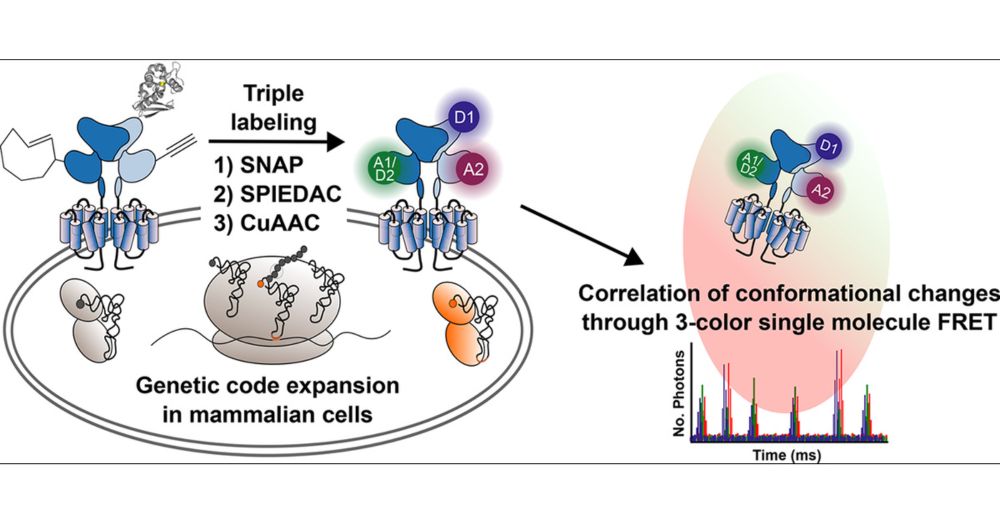
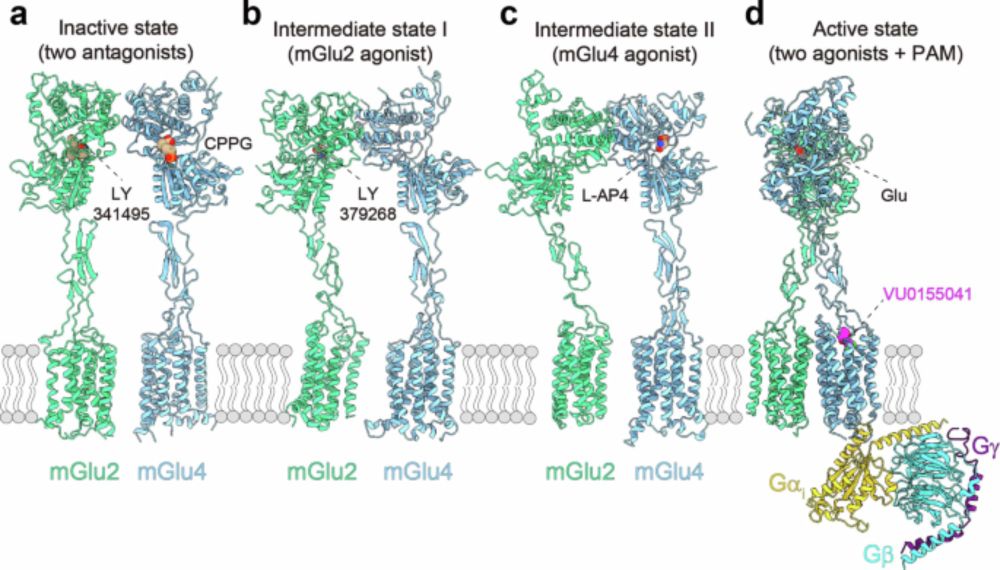
Biochemistry PI @CBS Montpellier working at the interfaces of biophysics, chemical and structural biology to unravel how structural dynamics control membrane protein function with particular focus on GPCRs.
Posts
Media
Videos
Starter Packs
Robert B. Quast
@rbquast.bsky.social
· Jan 22

Unlocking the Dynamics of GPCRs: Postdoctoral Researcher Opportunity in Structural Biology and Biophysics (M/F)
You will contribute to our pioneering efforts to dissect the structural dynamics of GPCRs using state-of-the-art tools and techniques.
euraxess.ec.europa.eu
Reposted by Robert B. Quast