Carole Duboc
@caroleduboc.bsky.social
130 followers
54 following
9 posts
Chemist
Bio-inorganic chemistry, Catalysis, Molecular chemistry, Electrocatalysis, Activation of small molecules, Mechanism, Spectroelectrochemistry,
CNRS Chimie
University Grenoble Alpes
Posts
Media
Videos
Starter Packs
Carole Duboc
@caroleduboc.bsky.social
· Jun 13
Carole Duboc
@caroleduboc.bsky.social
· Jun 13
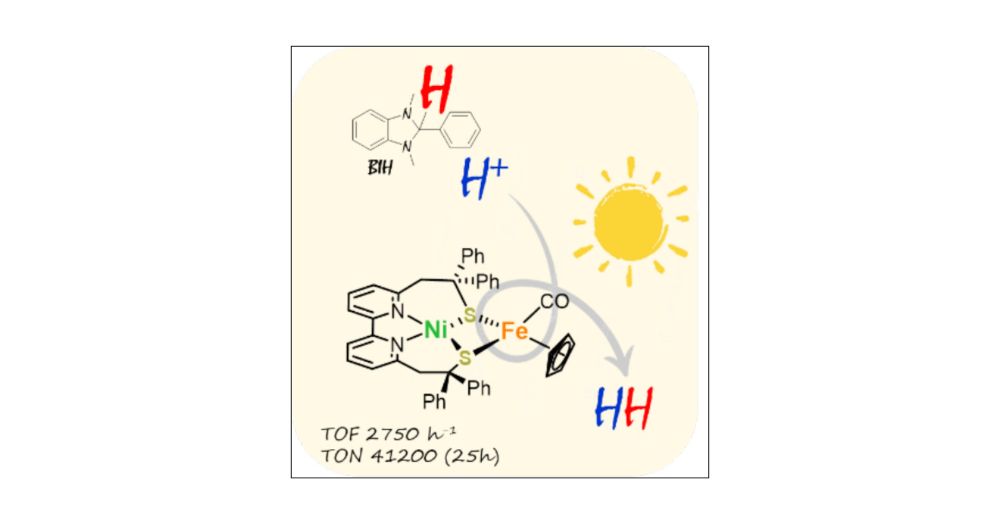
Unraveling Photo-Driven H2 Production with a Bio-Inspired NiFe Complex: Revealing an Unexpected Hydrogen Atom Source
Developing efficient photocatalytic systems for sustainable H2 production is a crucial step toward reducing dependence on fossil fuels. While extensive research over the past two decades has focused o...
pubs.acs.org
Carole Duboc
@caroleduboc.bsky.social
· Mar 4
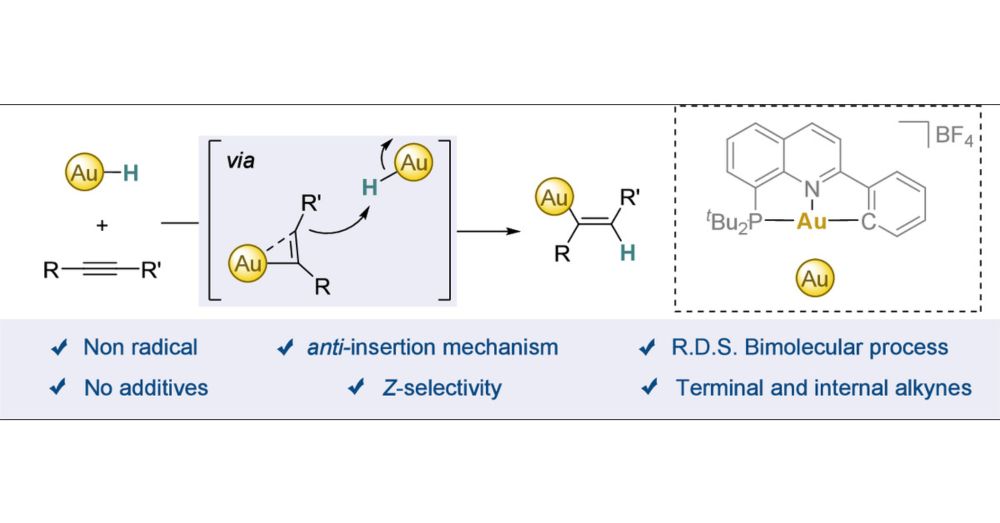
Interrogating the anti-Insertion of Alkynes into Gold(III)
Alkyne hydrofunctionalizations are a powerful strategy to efficiently build up structural complexity. The selectivity of these reactions is typically governed by the interaction between the alkyne and...
pubs.acs.org
Carole Duboc
@caroleduboc.bsky.social
· Mar 4
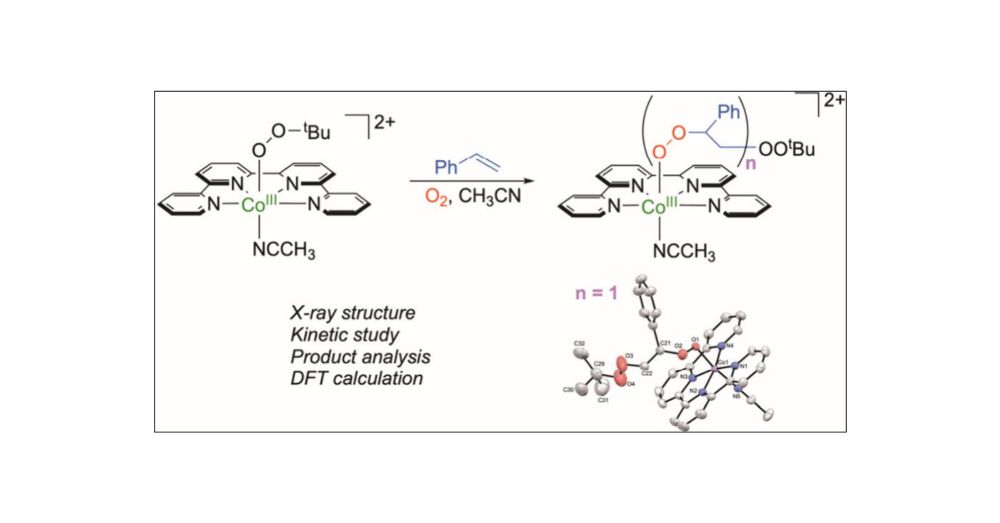
Selective Aerobic Peroxidation of Styrene Catalyzed by a Cobalt tert-Butylperoxo Complex
Selective oxidation of styrene to desired products is essential and challenging. In this study, we elucidate a unique pathway for the selective oxidation of styrene to polystyrene peroxo species, catalyzed by the cobalt(III) tert-butylperoxo complex, [CoIII(OOtBu)(qpy)(NCCH3)]2+ (1), under ambient conditions. Mechanistic investigations, including the structural determination of the diperoxo complex, [CoIII(qpy)(OOCH(Ph)CH2OOtBu)(NCCH3)]2+ (2), by X-ray analysis and theoretical calculations reveal that the reaction begins with the nucleophilic addition of styrene to the CoIII–OOtBu moiety in 1. This step is followed by an addition with an O2 molecule, forming a diperoxyl radical (PhCOO•(H)CH2OOtBu), which subsequently rebounds with CoII(qpy) to yield 2. In the presence of excess O2, complex 2 can further react with additional styrene molecules, leading to the formation of cobalt(III) polystyrene peroxo species.
pubs.acs.org
Carole Duboc
@caroleduboc.bsky.social
· Mar 4
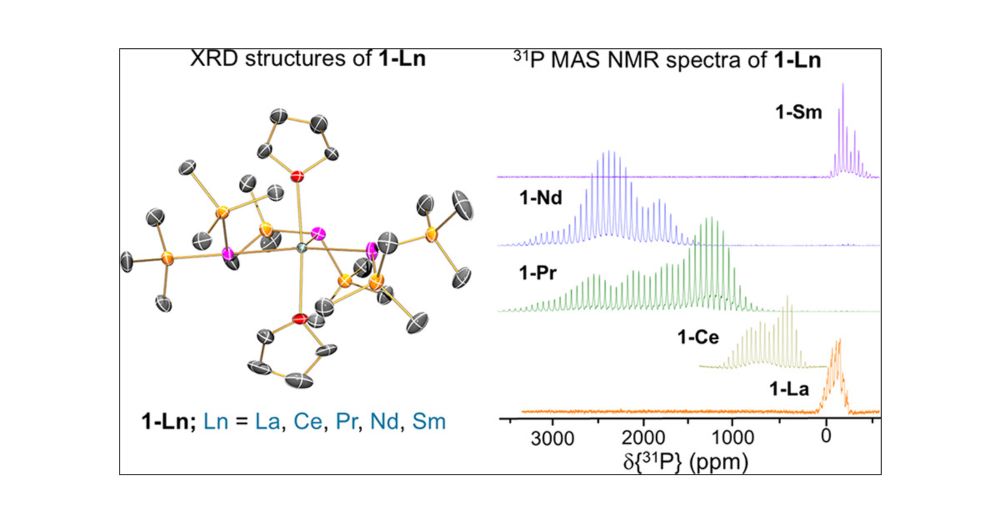
31P NMR Chemical Shift Anisotropy in Paramagnetic Lanthanide Phosphide Complexes
Lanthanide (Ln) magnetic resonance imaging and chiral shift reagents generally exploit 1H NMR shifts, as paramagnetic broadening tends to preclude the use of heavier, less sensitive nuclei. Here, we r...
pubs.acs.org
Reposted by Carole Duboc
Reposted by Carole Duboc
Reposted by Carole Duboc
Carole Duboc
@caroleduboc.bsky.social
· Feb 9
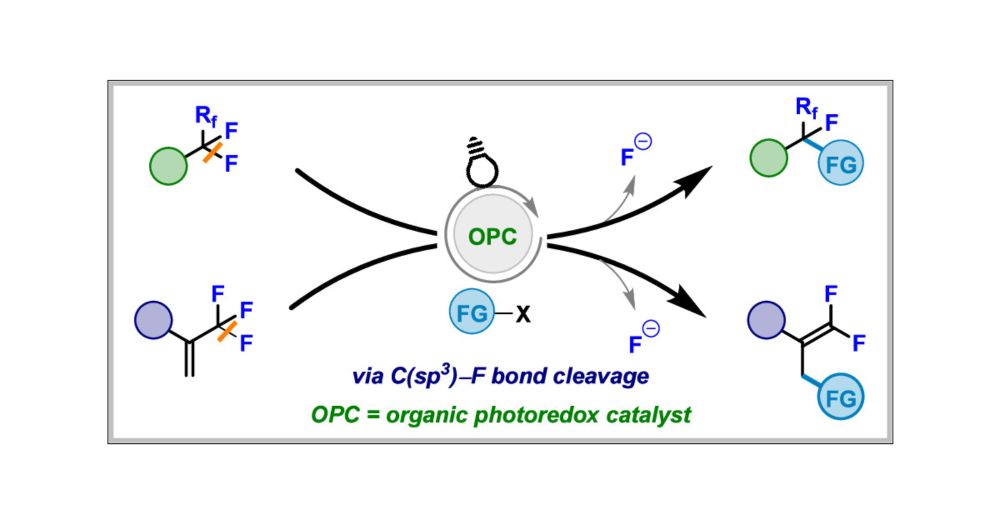
New Opportunities to Access Fluorinated Molecules Using Organophotoredox Catalysis via C(sp3)–F Bond Cleavage
Fluorinated molecules are of paramount importance because of their unique properties. As a result, the search for innovative approaches to the synthesis of this class of compounds has been relentless ...
doi.org
Reposted by Carole Duboc
Reposted by Carole Duboc
Reposted by Carole Duboc
Nature Chemistry
@natchem.nature.com
· Jan 21
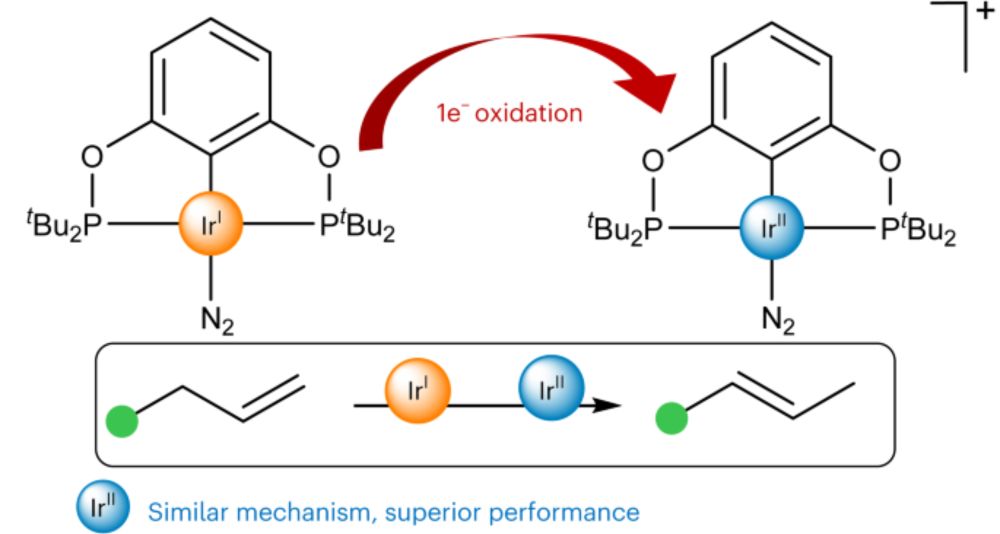
An open-shell Ir(II)/Ir(IV) redox couple outperforms an Ir(I)/Ir(III) pair in olefin isomerization - Nature Chemistry
The chemistry of precious-metal-based open-shell mononuclear complexes remains underdeveloped. Now it has been shown that iridium metalloradicals enable Ir(II)/Ir(IV) redox cycles and catalyse olefin ...
www.nature.com