Josh Lawrence
@jmlawrence.bsky.social
90 followers
120 following
38 posts
Research Fellow at Trinity Hall and Chemistry Department of the University of Cambridge | he/him
Posts
Media
Videos
Starter Packs
Pinned
Josh Lawrence
@jmlawrence.bsky.social
· Jul 21
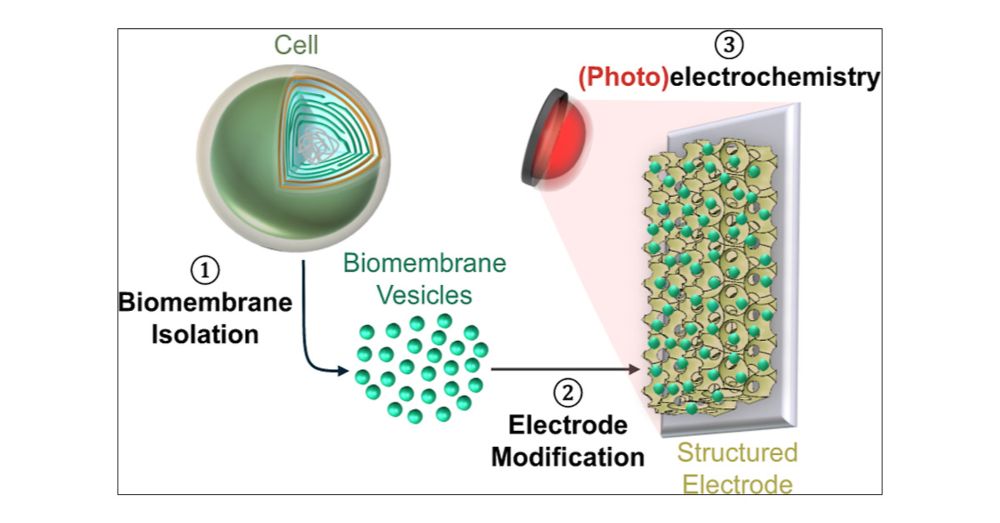
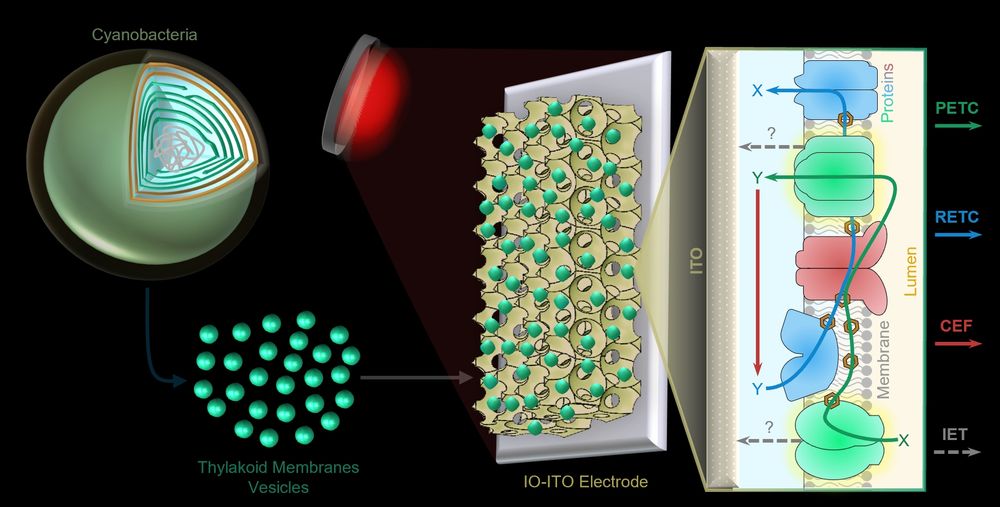
Dissecting Bioelectrical Networks in Photosynthetic Membranes with Electrochemistry
Photosynthetic membranes contain complex networks of redox proteins and molecules, which direct electrons along various energy-to-chemical interconversion reactions important for sustaining life on Ea...
pubs.acs.org
Josh Lawrence
@jmlawrence.bsky.social
· Jul 21
Josh Lawrence
@jmlawrence.bsky.social
· Jul 21

Dissecting Bioelectrical Networks in Photosynthetic Membranes with Electrochemistry
Photosynthetic membranes contain complex networks of redox proteins and molecules, which direct electrons along various energy-to-chemical interconversion reactions important for sustaining life on Ea...
pubs.acs.org
Reposted by Josh Lawrence
Leonid Digel
@leodigel.bsky.social
· Jul 4
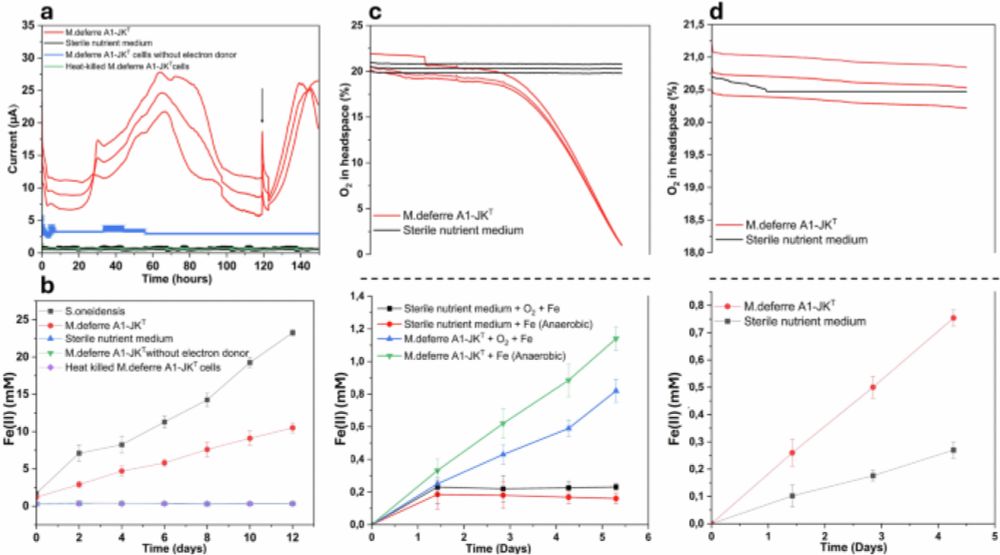
Iron reduction under oxic conditions by Microbacterium deferre sp. nov. A1-JKT - Nature Communications
In this study, the authors show that Microbacterium deferre A1-JK, a newly isolated Gram-positive bacterium, simultaneously reduces oxygen and iron under oxic conditions, revealing unexpected microbia...
www.nature.com
Josh Lawrence
@jmlawrence.bsky.social
· Mar 25
Josh Lawrence
@jmlawrence.bsky.social
· Mar 19
Josh Lawrence
@jmlawrence.bsky.social
· Mar 19
Josh Lawrence
@jmlawrence.bsky.social
· Mar 19
Josh Lawrence
@jmlawrence.bsky.social
· Mar 19
Josh Lawrence
@jmlawrence.bsky.social
· Mar 19
Josh Lawrence
@jmlawrence.bsky.social
· Mar 19