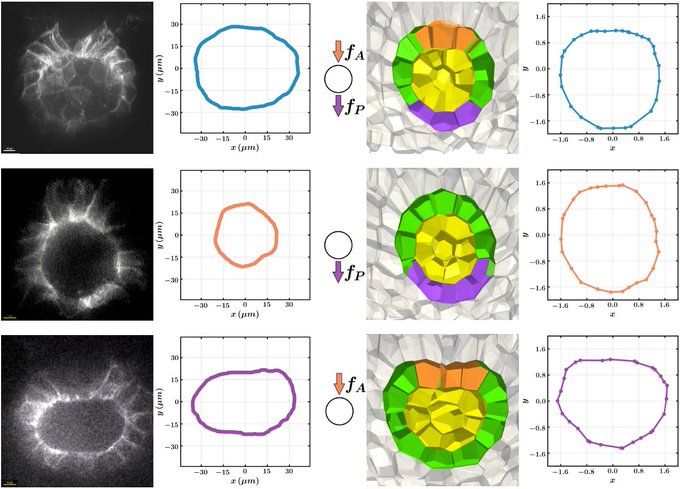
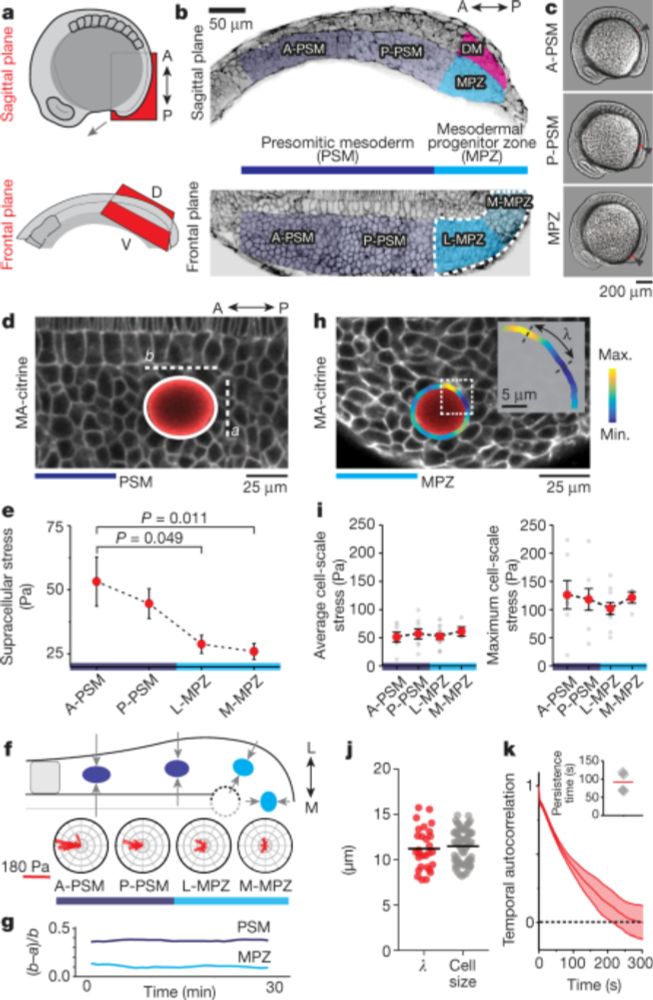
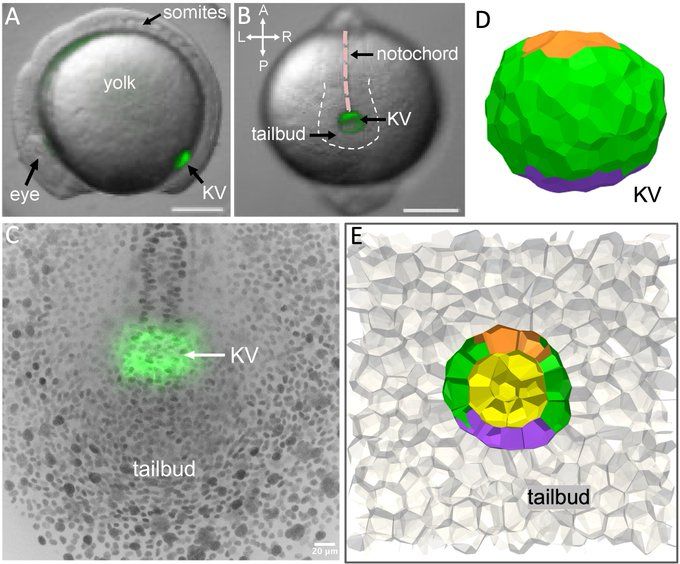
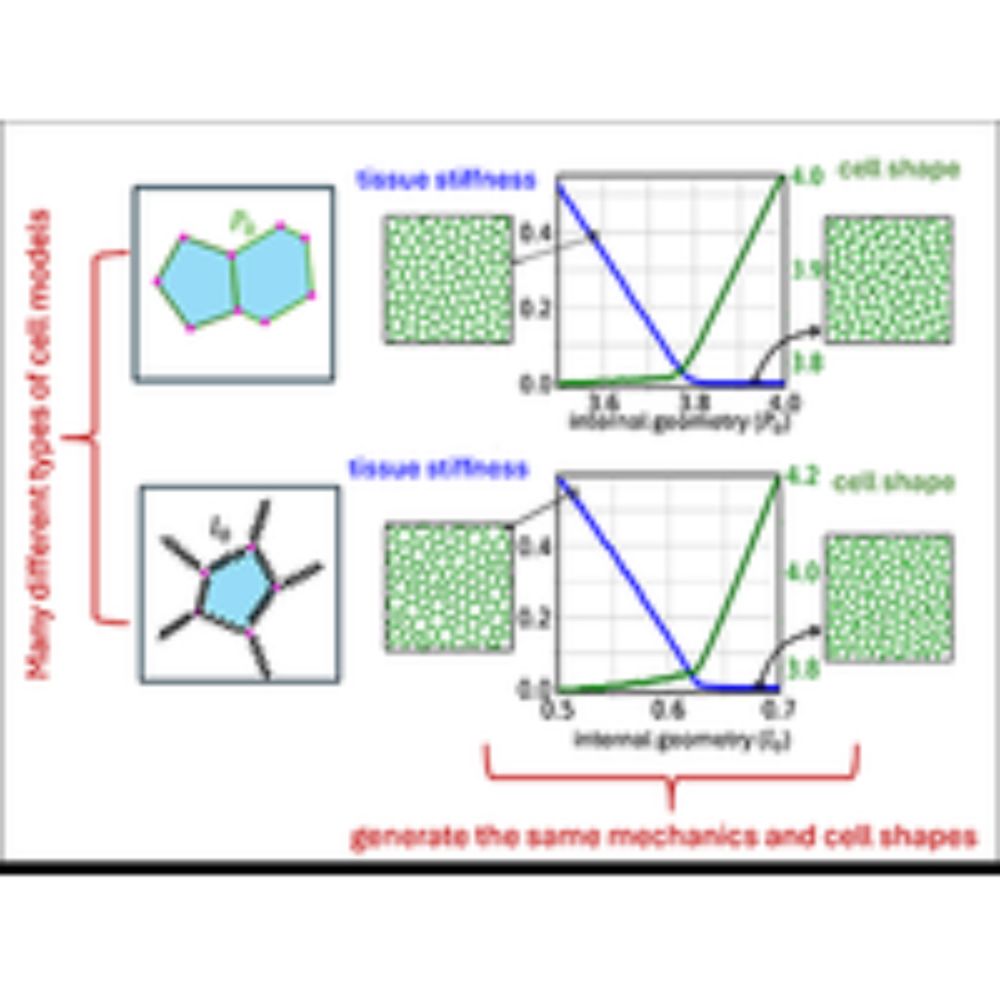
Manning Research Group
@manningresearch.bsky.social
150 followers
62 following
90 posts
Manning Research Group at Syracuse University: theory and computation focused on cells, grains, tissues, glasses, and other out-of-equilibrium disordered matter
Posts
Media
Videos
Starter Packs