Sadig Aghazada
@sadigaghazada.bsky.social
110 followers
91 following
12 posts
Asst. Prof. of Inorganic Chemistry at the University of Bern
Posts
Media
Videos
Starter Packs
Pinned
Sadig Aghazada
@sadigaghazada.bsky.social
· Feb 13
Reposted by Sadig Aghazada
Sadig Aghazada
@sadigaghazada.bsky.social
· Aug 21
L.G.
@gliam96.bsky.social
· Aug 21

Stepwise and reversible assembly of [2Fe–2S] rhombs to [8Fe–8S] clusters and their topological interconversions - Nature Chemistry
Elucidating the nature of the metallocofactors in nitrogenase enzymes, and preparing synthetic analogues of these clusters, is a classic target for bioinorganic chemists. Now the transformation of [Fe...
www.nature.com
Reposted by Sadig Aghazada
Reposted by Sadig Aghazada
Reposted by Sadig Aghazada
David Mills
@millsgroupchem.bsky.social
· Jun 25
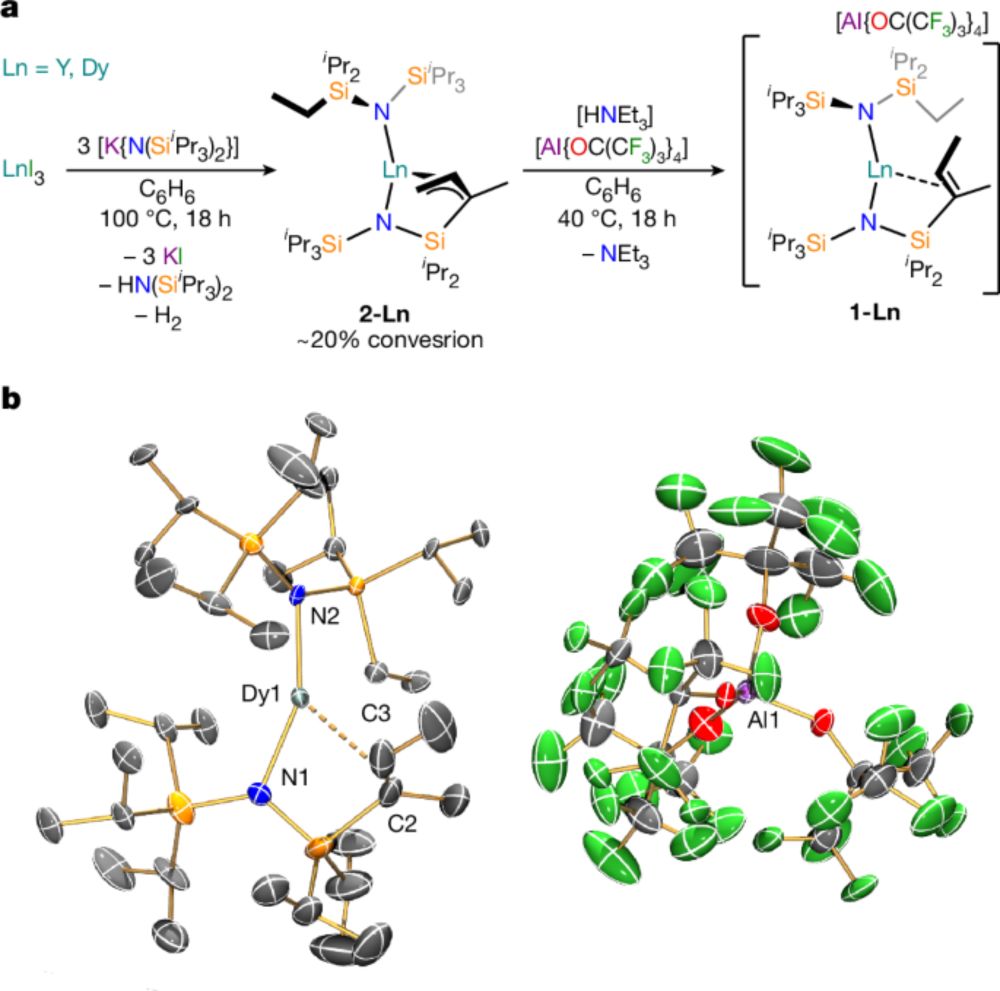
Soft magnetic hysteresis in a dysprosium amide–alkene complex up to 100 kelvin - Nature
A dysprosium amide–alkene complex shows soft magnetic hysteresis loops up to 100 kelvin, arising from the high charge density of the amide ligands and the structural role of the pendant alkene.
www.nature.com
Reposted by Sadig Aghazada
Reposted by Sadig Aghazada
Dominik Munz
@munzgroup.bsky.social
· Apr 26
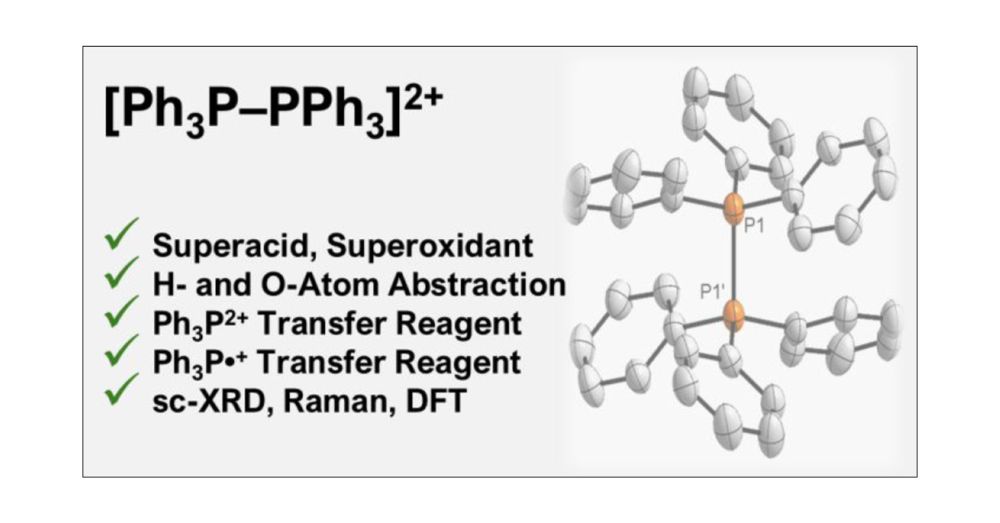
Hexaphenyl-1,2-Diphosphonium Dication [Ph3P–PPh3]2+: Superacid, Superoxidant, or Super Reagent?
The oxidation of triphenylphosphine by perfluorinated phenaziniumF aluminate in difluorobenzene affords hexaaryl-1,2-diphosphonium dialuminate 1. Dication 12+ is valence isoelectronic with elusive hex...
pubs.acs.org
Reposted by Sadig Aghazada
Sadig Aghazada
@sadigaghazada.bsky.social
· Feb 18
Reposted by Sadig Aghazada
Sadig Aghazada
@sadigaghazada.bsky.social
· Feb 14
Sadig Aghazada
@sadigaghazada.bsky.social
· Feb 14
Sadig Aghazada
@sadigaghazada.bsky.social
· Feb 14
Sadig Aghazada
@sadigaghazada.bsky.social
· Feb 14
Sadig Aghazada
@sadigaghazada.bsky.social
· Feb 13
Sadig Aghazada
@sadigaghazada.bsky.social
· Feb 13
Sadig Aghazada
@sadigaghazada.bsky.social
· Feb 13
Sadig Aghazada
@sadigaghazada.bsky.social
· Feb 13
Reposted by Sadig Aghazada
Schneider Lab
@schneider-lab.bsky.social
· Jan 29
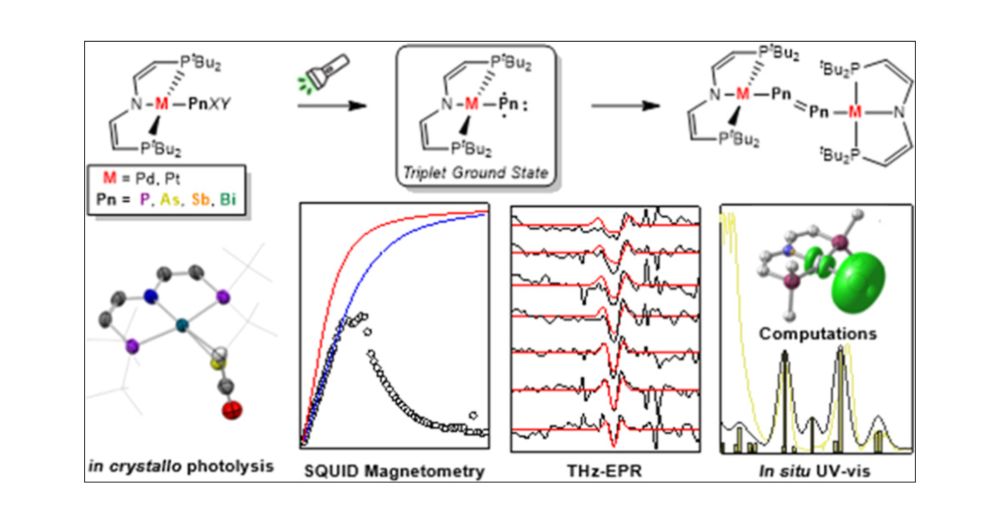
Transient Triplet Metallopnictinidenes M–Pn (M = PdII, PtII; Pn = P, As, Sb): Characterization and Dimerization
Nitrenes (R–N) have been subject to a large body of experimental and theoretical studies. The fundamental reactivity of this important class of transient intermediates has been attributed to their electronic structures, particularly the accessibility of triplet vs singlet states. In contrast, electronic structure trends along the heavier pnictinidene analogues (R–Pn; Pn = P–Bi) are much less systematically explored. We here report the synthesis of a series of metallodipnictenes, {M–Pn═Pn–M} (M = PdII, PtII; Pn = P, As, Sb, Bi) and the characterization of the transient metallopnictinidene intermediates, {M–Pn} for Pn = P, As, Sb. Structural, spectroscopic, and computational analysis revealed spin triplet ground states for the metallopnictinidenes with characteristic electronic structure trends along the series. In comparison to the nitrene, the heavier pnictinidenes exhibit lower-lying ground state SOMOs and singlet excited states, thus suggesting increased electrophilic reactivity. Furthermore, the splitting of the triplet magnetic microstates is beyond the phosphinidenes {M–P} dominated by heavy pnictogen atom induced spin–orbit coupling.
pubs.acs.org