Andrew Boggiano
@acboggiano.bsky.social
77 followers
110 following
21 posts
Postdoc at UW Madison Cyclotron Group. PhD from Georgia Tech. Synthetic inorganic chemist, lanthanides, actinides. Single-crystal XRD for life. 🏳️🌈
Posts
Media
Videos
Starter Packs
Andrew Boggiano
@acboggiano.bsky.social
· Apr 24
Reposted by Andrew Boggiano
Andrew Boggiano
@acboggiano.bsky.social
· Apr 17
Reposted by Andrew Boggiano
Reposted by Andrew Boggiano
Reposted by Andrew Boggiano
John Seed
@john-seed.bsky.social
· Apr 11
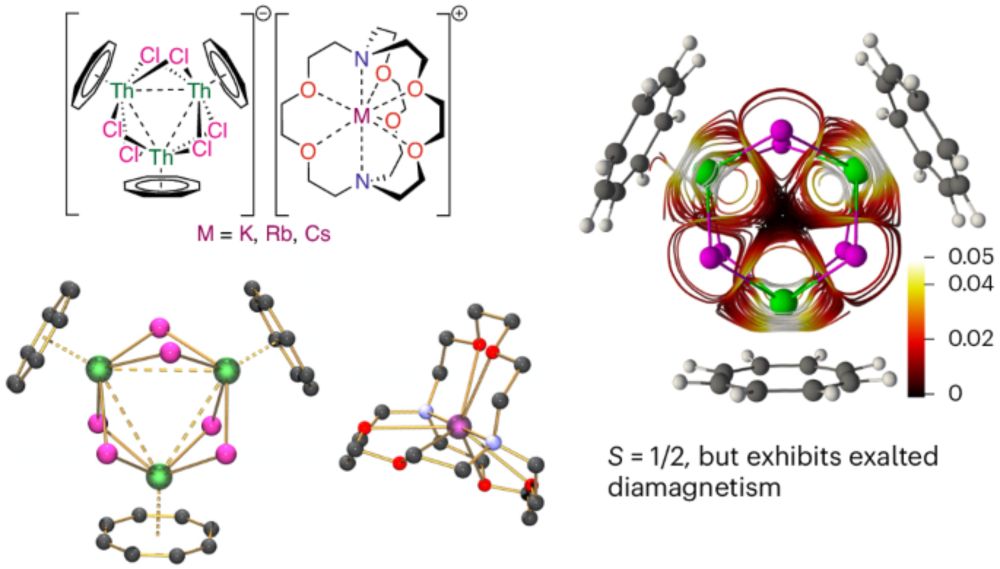
Valence-delocalized trithorium nanocluster superatoms with open-shell exalted diamagnetism - Nature Chemistry
Superatoms are metal clusters that collectively behave like an atom, but they usually require metal–metal bonding and thus they are based on main group or transition metals. Now it has been shown that...
www.nature.com
Andrew Boggiano
@acboggiano.bsky.social
· Apr 11
Reposted by Andrew Boggiano
Reposted by Andrew Boggiano
Reposted by Andrew Boggiano
Reposted by Andrew Boggiano
Andrew Boggiano
@acboggiano.bsky.social
· Jan 31
Reposted by Andrew Boggiano
Andrew Boggiano
@acboggiano.bsky.social
· Jan 27
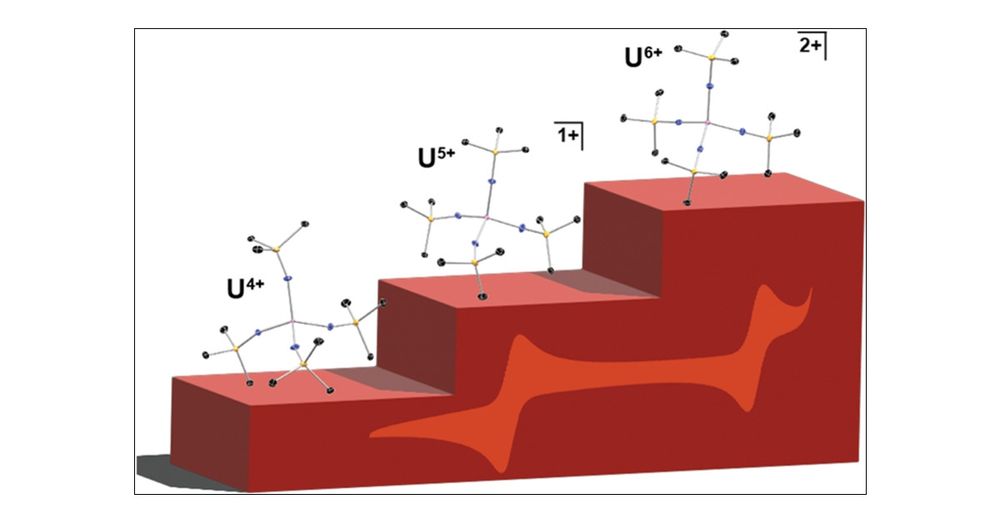
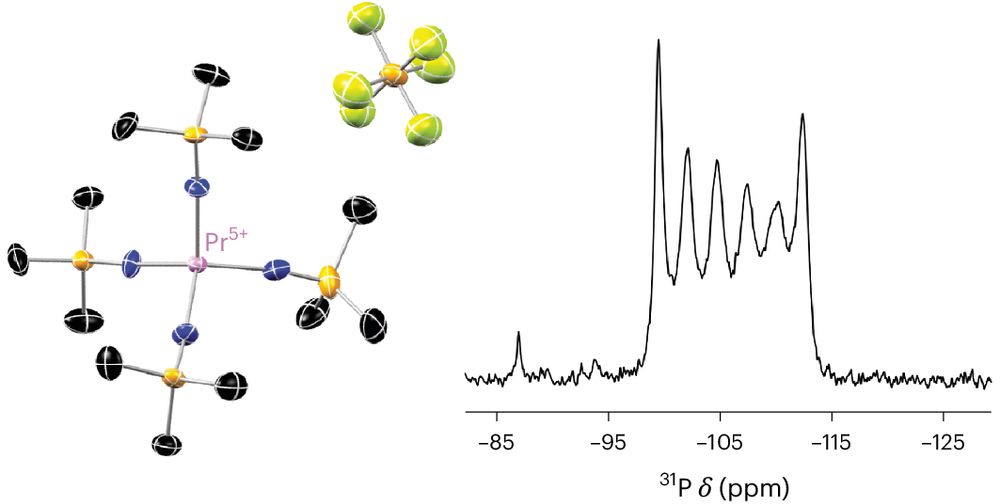
U4+/5+/6+ in a Conserved Pseudotetrahedral Imidophosphorane Coordination Sphere
While several ligand systems support uranium across a range of oxidation states, spanning more than two oxidation states in a conserved coordination geometry is uncommon among structurally authenticated complexes. Imidophosphorane ligands significantly stabilize high-valent lanthanide and actinide complexes. Here, we report a series of homoleptic uranium imidophosphorane complexes, spanning the +4, +5 and +6 oxidation states in a four-coordinate pseudotetrahedral ligand field. The +6 oxidation state is accessible using a mild ferrocenium oxidant, yielding a rare example of U6+ in a pseudotetrahedral coordination environment. As the formal oxidation state increases, the U–N distances gradually contract, consistent with the Shannon ionic radii of U4+/5+/6+. Compared to reported complexes, the short U–N distances observed in the U6+ complex are more comparable to dianionic imido ligands than monoanionic amido ligands.
pubs.acs.org
Andrew Boggiano
@acboggiano.bsky.social
· Jan 16
Reposted by Andrew Boggiano