Christoph Schran
@cschran.bsky.social
120 followers
70 following
6 posts
Assistant Professor @ Cavendish Laboratory, University of Cambridge
Group leader of the FAST group: https://www.fast-group.phy.cam.ac.uk/
Posts
Media
Videos
Starter Packs
Christoph Schran
@cschran.bsky.social
· May 7
FAST Group
@fast-group.bsky.social
· Apr 29
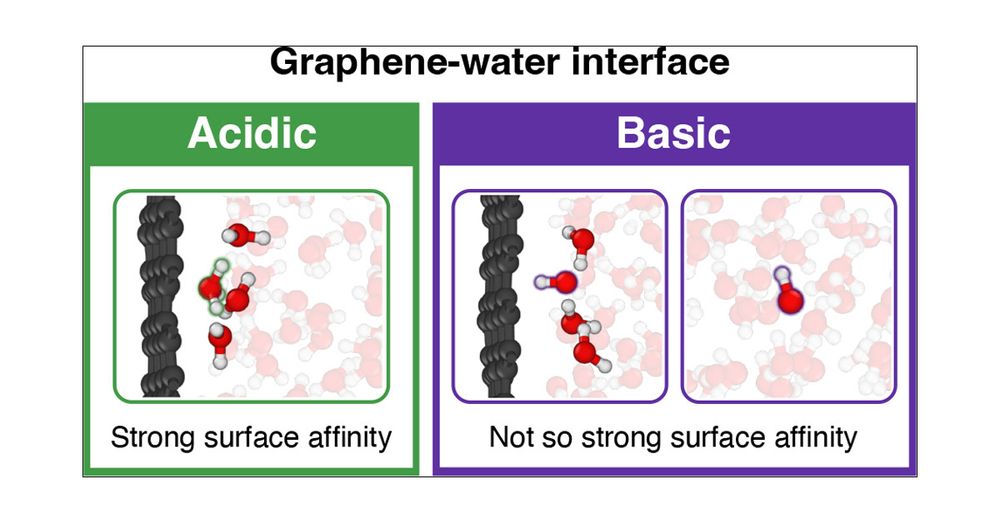
Protons Accumulate at the Graphene–Water Interface
Water’s ability to autoionize into hydroxide and hydronium ions profoundly influences surface properties, rendering interfaces either basic or acidic. While it is well-established that protons show an affinity to the air–water interface, a critical knowledge gap exists in technologically relevant surfaces like the graphene–water interface. Here we use machine learning-based simulations with first-principles accuracy to unravel the behavior of hydroxide and hydronium ions at the graphene–water interface. Our findings reveal that protons accumulate at the graphene–water interface, with the hydronium ion predominantly residing in the first contact layer of water. In contrast, the hydroxide ion exhibits a bimodal distribution, found both near the surface and further away from it. Analysis of the underlying electronic structure reveals local polarization effects, resulting in counterintuitive charge rearrangement. Proton propensity to the graphene–water interface challenges the interpretation of surface experiments and is expected to have far-reaching consequences for ion conductivity, interfacial reactivity, and proton-mediated processes.
doi.org
Reposted by Christoph Schran
Christoph Schran
@cschran.bsky.social
· Dec 4
Christoph Schran
@cschran.bsky.social
· Dec 3