- week 8 induction data presented at #UEGWeek
- first in class for IBD
1/🧵
MoA: ⬆️expression of miR-124, which regulates inflammatory response & restores mucosal homeostasis.

- week 8 induction data presented at #UEGWeek
- first in class for IBD
1/🧵
MoA: ⬆️expression of miR-124, which regulates inflammatory response & restores mucosal homeostasis.
1/ #IBD is rising fast in SE Asia 📈
We need tools that are accurate, patient-friendly, and sustainable.
Our SGH pilot study shows #IntestinalUltrasound ticks all 3 boxes ✅

1/ #IBD is rising fast in SE Asia 📈
We need tools that are accurate, patient-friendly, and sustainable.
Our SGH pilot study shows #IntestinalUltrasound ticks all 3 boxes ✅
A phase 2a induction trial of tulisokibart, an anti-TL1A monoclonal antibody, shows promising efficacy & safety in patients with moderate-to-severe Crohn’s disease (CD) refractory to prior therapies.
Key findings from the APOLLO-CD study:
[🔗https://doi.org/10.1016/S2468-1253(25)00071-8]
A phase 2a induction trial of tulisokibart, an anti-TL1A monoclonal antibody, shows promising efficacy & safety in patients with moderate-to-severe Crohn’s disease (CD) refractory to prior therapies.
Key findings from the APOLLO-CD study:
[🔗https://doi.org/10.1016/S2468-1253(25)00071-8]
Crohn's: journals.lww.com/ajg/fulltext...
UC: journals.lww.com/ajg/fulltext...
#IBDSky #MedSky #GISky




Crohn's: journals.lww.com/ajg/fulltext...
UC: journals.lww.com/ajg/fulltext...
#IBDSky #MedSky #GISky
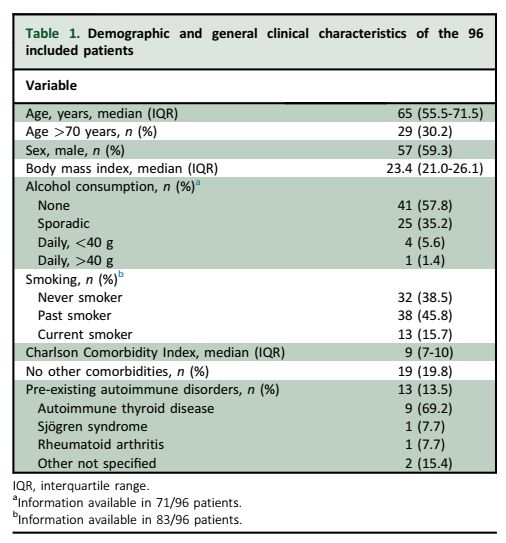
1/ ICI-induced colitis
📈 Affects up to 30% of patients on ICIs.
🔬 This study followed 96 patients with 12-month outcomes.
www.esmoopen.com/article/S205...
1/ ICI-induced colitis
📈 Affects up to 30% of patients on ICIs.
🔬 This study followed 96 patients with 12-month outcomes.
www.esmoopen.com/article/S205...




gut.bmj.com/content/earl...
@bmj.com #GISky #IBDSky




gut.bmj.com/content/earl...
@bmj.com #GISky #IBDSky
Score of ≥3 was 84% predictive of steroid failure
- CRP ≥100mg/L (1 point)
- albumin ≤25g/L (1 point)
- UCEIS ≥4 (1 point) or ≥7 (2 points)
Will these pts benefit from early 'salvage' therapy instead of waiting till Day 3?
#IBDSky #GISky
gut.bmj.com/content/72/3...


Score of ≥3 was 84% predictive of steroid failure
- CRP ≥100mg/L (1 point)
- albumin ≤25g/L (1 point)
- UCEIS ≥4 (1 point) or ≥7 (2 points)
Will these pts benefit from early 'salvage' therapy instead of waiting till Day 3?
#IBDSky #GISky
gut.bmj.com/content/72/3...
4 parameters:
- size of ulcers
- extent of ulcerated surface
- extent of colitis
- stenosis
5 segments:
- ileum, right, transverse, left colon and rectum
Endo remission: ≤2 (or <4)
Accessible online calculator: www.e-guide.ecco-ibd.eu/resources/ca...
#IBDSky #GISky #SurgSky

4 parameters:
- size of ulcers
- extent of ulcerated surface
- extent of colitis
- stenosis
5 segments:
- ileum, right, transverse, left colon and rectum
Endo remission: ≤2 (or <4)
Accessible online calculator: www.e-guide.ecco-ibd.eu/resources/ca...
#IBDSky #GISky #SurgSky
Guselkumab is an IL-23 inhibitor.
GRAVITI tested fully SC dosing (no IV) for both induction & maintenance in moderate-to-severe Crohn’s.
N = 347 adults, randomized 1:1:1
- GUS 400 mg q4w induction → 100 mg q8w
- GUS 400 mg q4w induction → 200 mg q4w
#IBDSky #GISky #MedSky



The #ACCURE trial says: Yes. 🧵
✂️ Pragmatic, open-label RCT across 22 centers (Netherlands, UK, Ireland).
Patients in UC remission randomized to:
✅ Appendicectomy + standard medical therapy
vs
🧪 Standard medical therapy alone


The #ACCURE trial says: Yes. 🧵
✂️ Pragmatic, open-label RCT across 22 centers (Netherlands, UK, Ireland).
Patients in UC remission randomized to:
✅ Appendicectomy + standard medical therapy
vs
🧪 Standard medical therapy alone
UST group has 90% who are bio-experienced compared to VDZ and anti-TNF group (30+%), leading to:
1. High 'persistence' cos lack of options
2. 'Lower' efficacy of UST
#IBDSky #GISky
academic.oup.com/ecco-jcc/adv...



UST group has 90% who are bio-experienced compared to VDZ and anti-TNF group (30+%), leading to:
1. High 'persistence' cos lack of options
2. 'Lower' efficacy of UST
#IBDSky #GISky
academic.oup.com/ecco-jcc/adv...
- FIT is non-inferior to colonoscopy for CRC-related deaths at 10 years
n= 57404
Participation rates higher for FIT (39.9%) vs scope (31.8%)
CRC-deaths similar: FIT (0.24%) vs scope (0.22%)
#GISky #SurgSky
@thelancet.bsky.social
www.thelancet.com/journals/lan...




- FIT is non-inferior to colonoscopy for CRC-related deaths at 10 years
n= 57404
Participation rates higher for FIT (39.9%) vs scope (31.8%)
CRC-deaths similar: FIT (0.24%) vs scope (0.22%)
#GISky #SurgSky
@thelancet.bsky.social
www.thelancet.com/journals/lan...
- higher risk of colorectal Ca, biliary Ca and cirrhosis
- dx: MRC, histology, ALP/GGT, IBD
- no proven therapy, UDCA can be considered for high-risk patients
#LiverSky #GISky
www.nature.com/articles/s41...




- higher risk of colorectal Ca, biliary Ca and cirrhosis
- dx: MRC, histology, ALP/GGT, IBD
- no proven therapy, UDCA can be considered for high-risk patients
#LiverSky #GISky
www.nature.com/articles/s41...
TB:
- necrotizing (caseating), >400 microns, multiple (>5/hpf), submucosa, confluent
Crohn's:
- non-necrotizing, <200 microns, infrequent, mucosa, non-confluent
tl;dr: TB granulomas are larger, 'deeper' and more extensive.
#IBDSky #GISky #IDSky



Crohn's
1st line: anti-TNF (+IM), RZK
2nd line: RZK, UPA
UC
1st line: VDZ, IFX, anti-p19
2nd line: UPA, TOFA, IFX, UST, anti-p19
@amergastroassn.bsky.social
#IBDSky #GISky



Crohn's
1st line: anti-TNF (+IM), RZK
2nd line: RZK, UPA
UC
1st line: VDZ, IFX, anti-p19
2nd line: UPA, TOFA, IFX, UST, anti-p19
@amergastroassn.bsky.social
#IBDSky #GISky
- most are permanent (low rates of successful reversal)
- some go on to have proctectomy for perianal disease
- successful reversal is a/w anti-TNF treatment
#IBDSky #SurgSky
pubmed.ncbi.nlm.nih.gov/39887905/



- most are permanent (low rates of successful reversal)
- some go on to have proctectomy for perianal disease
- successful reversal is a/w anti-TNF treatment
#IBDSky #SurgSky
pubmed.ncbi.nlm.nih.gov/39887905/
- IBD vs non-IBD surgery
- biologics vs small molecules
- risk of complications
- urgency of surgery
- post-op IBD treatment plans
#GISky #IBDSky #SurgSky
www.cghjournal.org/article/S154...
@amergastroassn.bsky.social




- IBD vs non-IBD surgery
- biologics vs small molecules
- risk of complications
- urgency of surgery
- post-op IBD treatment plans
#GISky #IBDSky #SurgSky
www.cghjournal.org/article/S154...
@amergastroassn.bsky.social
- anti-integrin and anti-cytokines classified as "high risk" (>10% HBVr in HBsAg+)
- VDZ and UST would be "high risk"
--> but VDZ classified as "gut specific anti-T cell rx"
--> did not expect UST to be "high risk"
#IBDSky #LiverSky




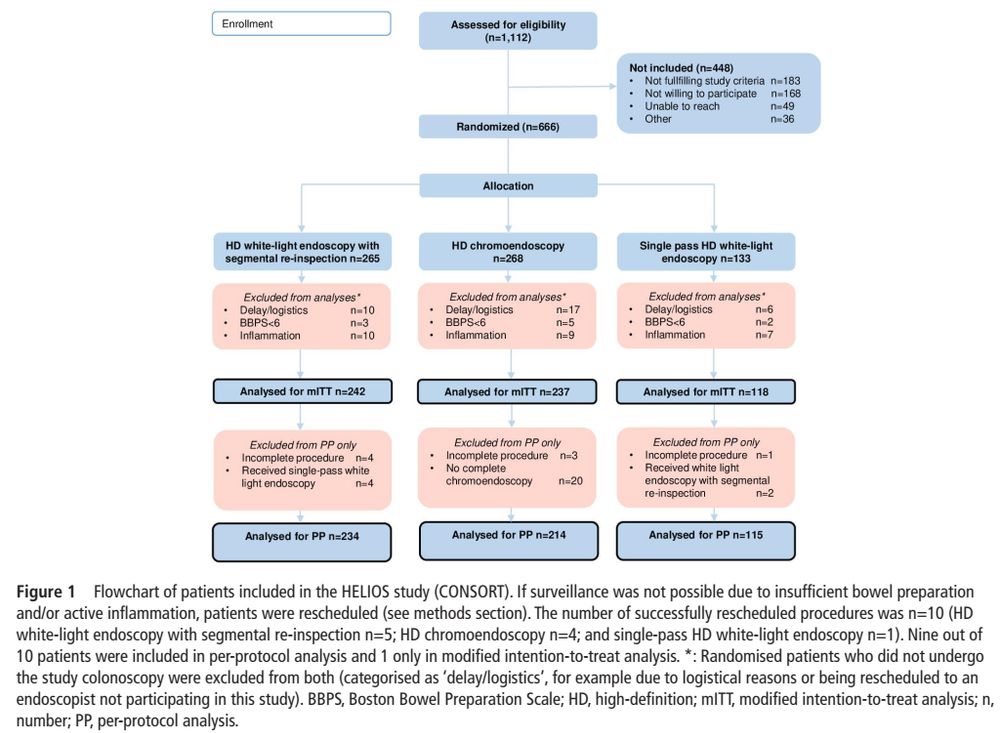
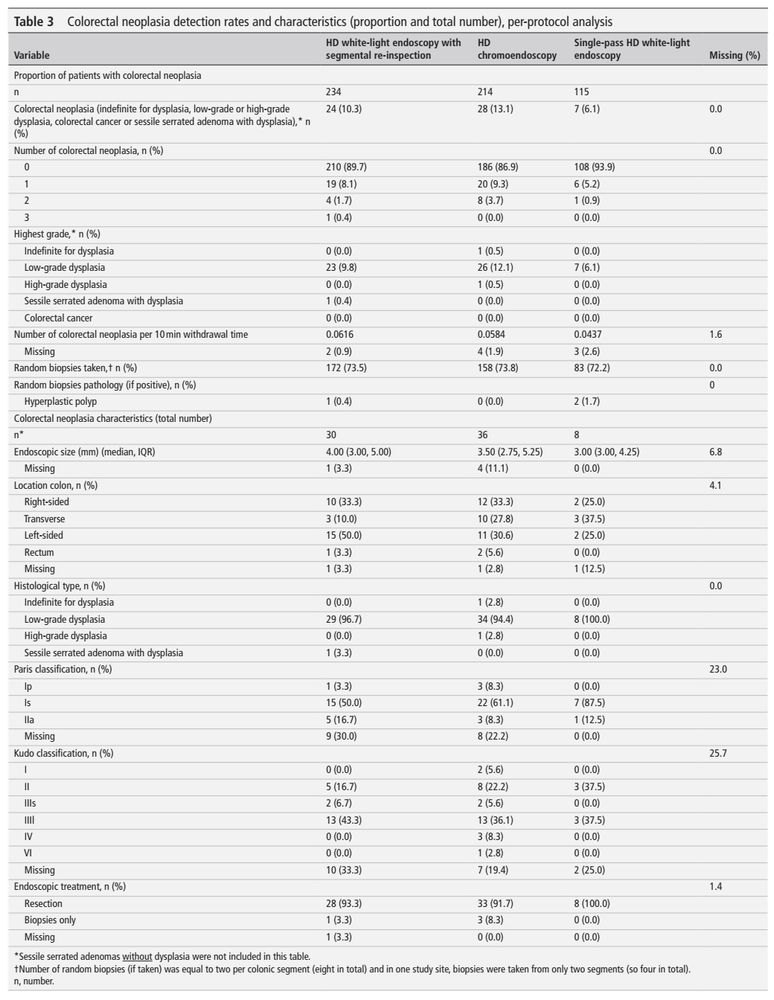
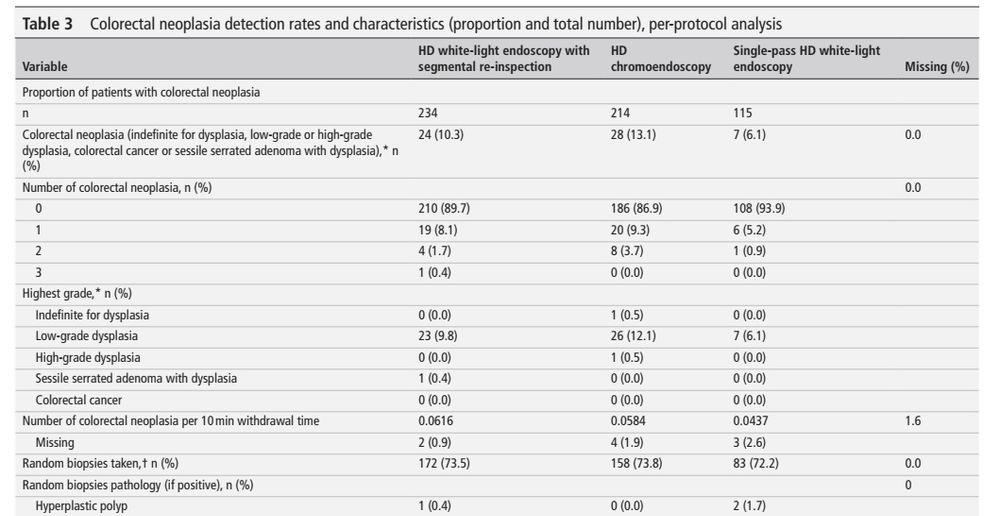
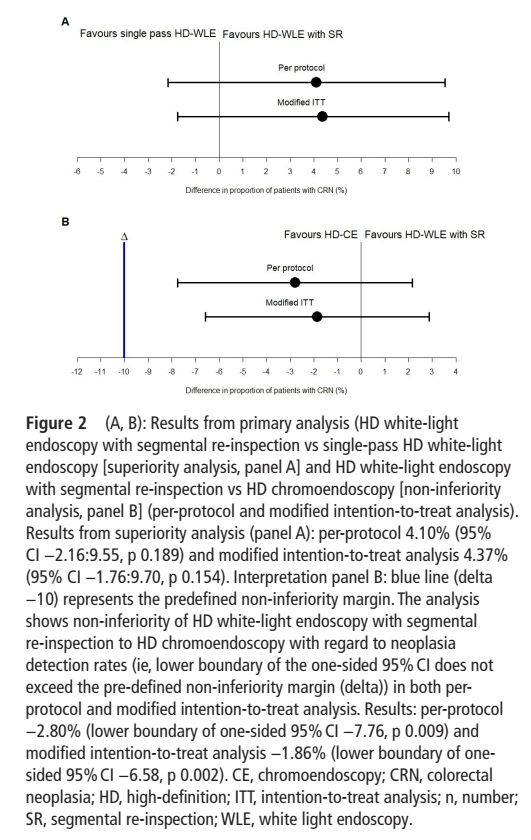
- high-def segmental re-inspection is not inferior to high-def chromoendoscopy (10.3% vs 13.1%, non-inferiority margin of 10%)
- 'benefit' of chromoendoscopy might be due to withdrawal/inspection time
- overall neoplasia rate was low (10.5%)
#IBDSky
- high-def segmental re-inspection is not inferior to high-def chromoendoscopy (10.3% vs 13.1%, non-inferiority margin of 10%)
- 'benefit' of chromoendoscopy might be due to withdrawal/inspection time
- overall neoplasia rate was low (10.5%)
#IBDSky






