
Group website: https://etosgroup.fi/

#chemsky #scisky #moty2025
cen.acs.org/synthesis/Mo...
#chemsky #scisky #moty2025
cen.acs.org/synthesis/Mo...
#ChemSky
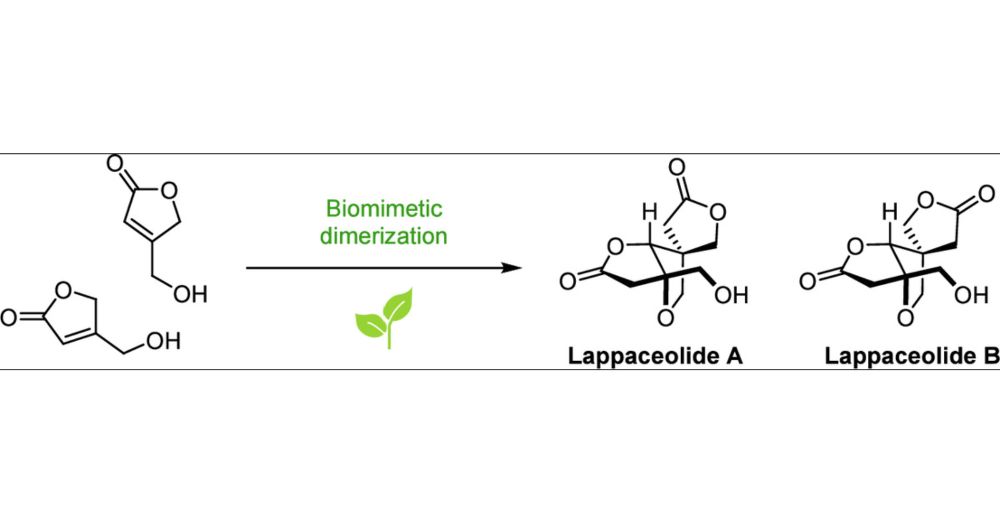
pubs.acs.org/doi/10.1021/...

#ChemSky
pubs.acs.org/doi/10.1021/...
pubs.acs.org/doi/10.1021/...
pubs.acs.org/doi/10.1021/...

Finnish Research Council is about to announce a special call to fund researchers abroad to relocate to Finland.
By all means this is not intended to lure you into moving to Finland, it just happens to be planned right now by pure chance.
Finnish Research Council is about to announce a special call to fund researchers abroad to relocate to Finland.
By all means this is not intended to lure you into moving to Finland, it just happens to be planned right now by pure chance.
Deadline: 18th April 2025.
elxw.fa.em3.oraclecloud.com/hcmUI/Candid...
Deadline: 18th April 2025.
elxw.fa.em3.oraclecloud.com/hcmUI/Candid...
1. Machine Learning-Guided Synthetic Strategies for Natural Product Synthesis, with @king-smithgroup.bsky.social
www.findaphd.com/phds/project...
2. Biomimetic Total Synthesis of Natural Products
www.findaphd.com/phds/project...

1. Machine Learning-Guided Synthetic Strategies for Natural Product Synthesis, with @king-smithgroup.bsky.social
www.findaphd.com/phds/project...
2. Biomimetic Total Synthesis of Natural Products
www.findaphd.com/phds/project...
@uniuef.bsky.social, @elsevierconnect.bsky.social, #chemsky - Check it out - doi.org/10.1016/j.ej...

@uniuef.bsky.social, @elsevierconnect.bsky.social, #chemsky - Check it out - doi.org/10.1016/j.ej...
@aalto.fi #chemsky
doi.org/10.1002/chem...

@aalto.fi #chemsky
doi.org/10.1002/chem...

- Computer Architecture
- Programming Languages
- Human-Computer Interaction
- Software Engineering
- Systems Security
www.aalto.fi/en/departmen...

- Computer Architecture
- Programming Languages
- Human-Computer Interaction
- Software Engineering
- Systems Security
www.aalto.fi/en/departmen...

Part 2 took a while, though! adventofcode.com/2024/day/12
Part 2 took a while, though! adventofcode.com/2024/day/12



@petripihko.bsky.social confirmed that the problem is just not at my end. Has anyone been able to fix this?

@petripihko.bsky.social confirmed that the problem is just not at my end. Has anyone been able to fix this?



