Kirill Sechkar
@ksechkar.bsky.social
41 followers
70 following
51 posts
DPhil Engineering Science student at Oxford Uni. All opinions are my own, but the risk you take by reading them is yours
Other soc. media: http://cosoc.com/KSechkar
Posts
Media
Videos
Starter Packs
Pinned
Kirill Sechkar
@ksechkar.bsky.social
· Apr 29
Kirill Sechkar
@ksechkar.bsky.social
· Apr 29
Kirill Sechkar
@ksechkar.bsky.social
· Apr 29
Kirill Sechkar
@ksechkar.bsky.social
· Apr 29
Kirill Sechkar
@ksechkar.bsky.social
· Apr 29
Reposted by Kirill Sechkar
Kirill Sechkar
@ksechkar.bsky.social
· Apr 4
Kirill Sechkar
@ksechkar.bsky.social
· Apr 4
Kirill Sechkar
@ksechkar.bsky.social
· Apr 4
Kirill Sechkar
@ksechkar.bsky.social
· Apr 4

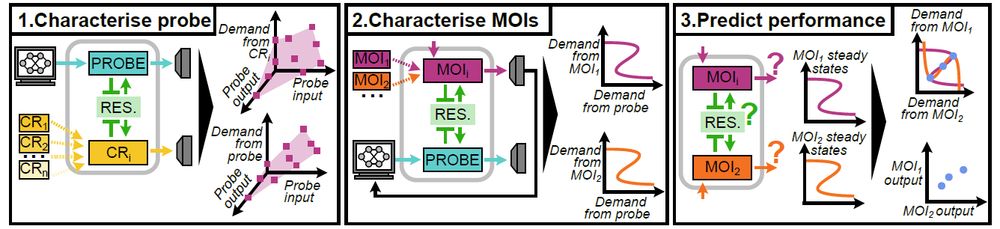
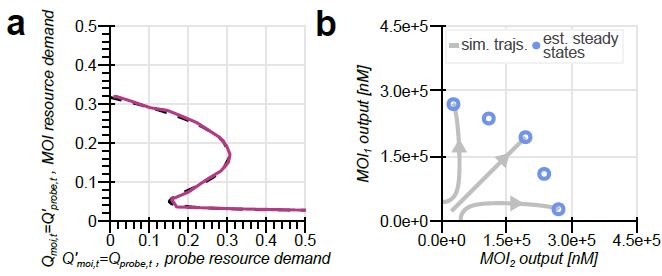
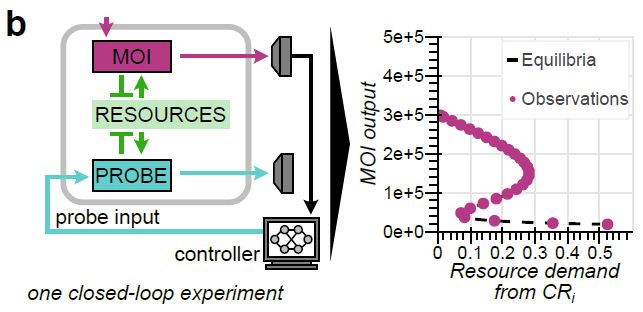
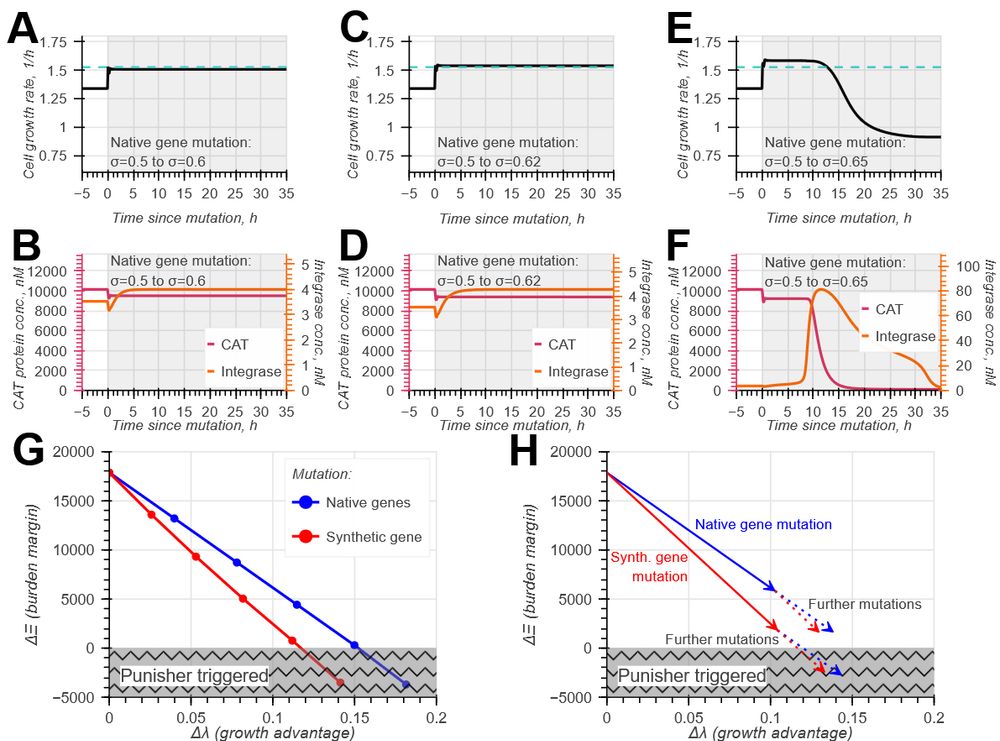
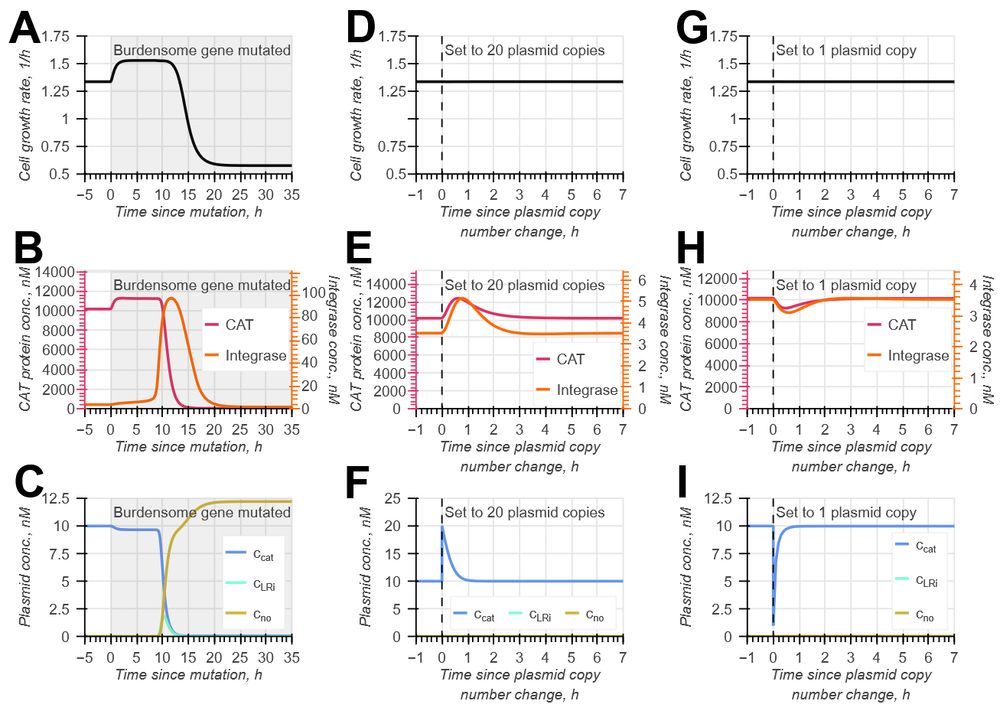
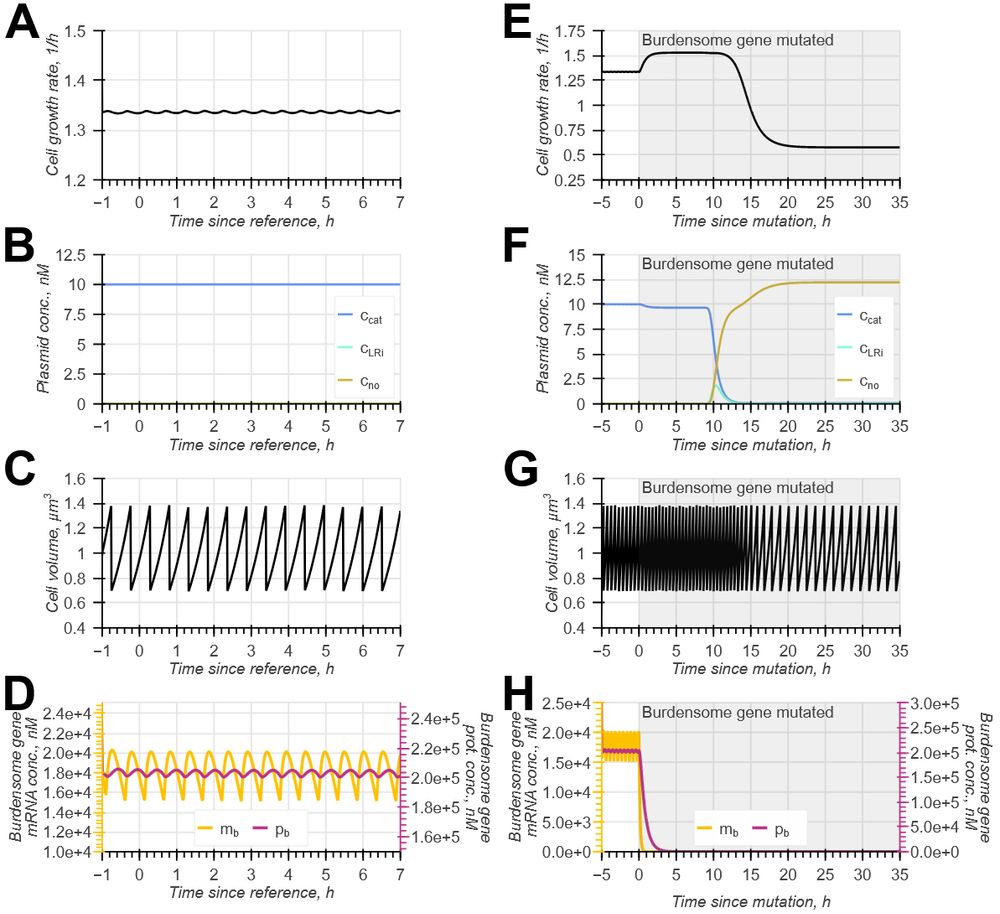
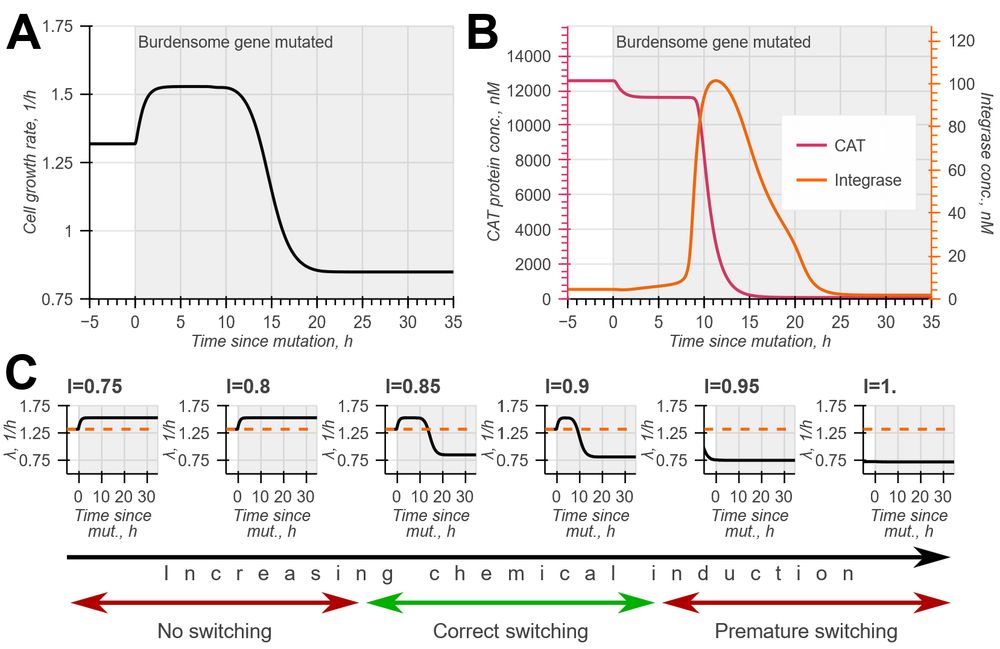
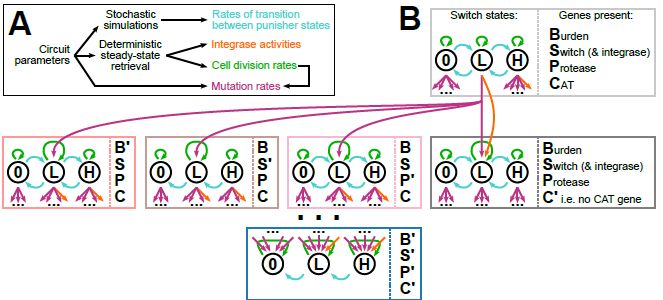
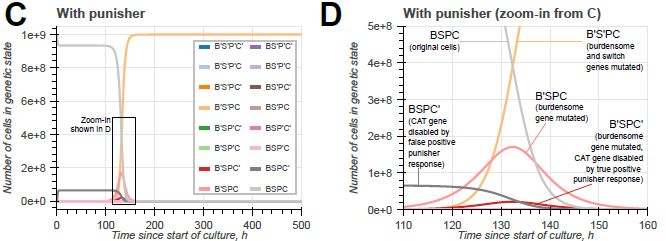
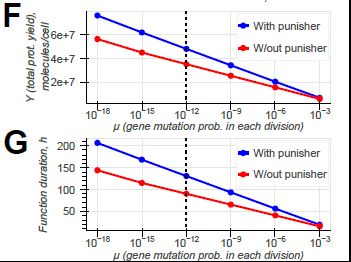
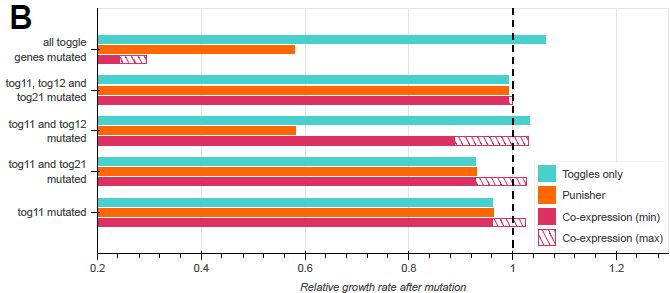
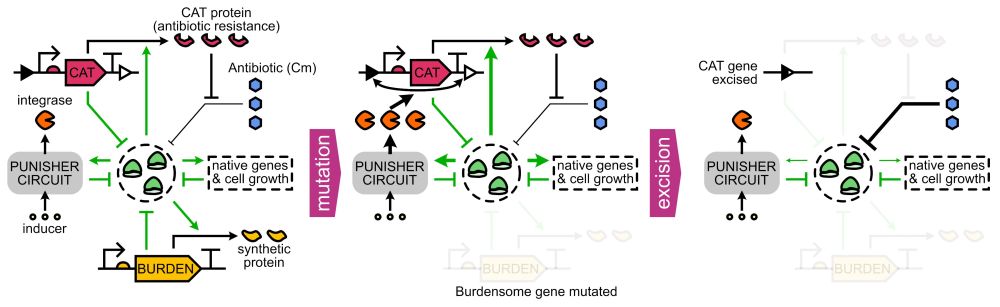
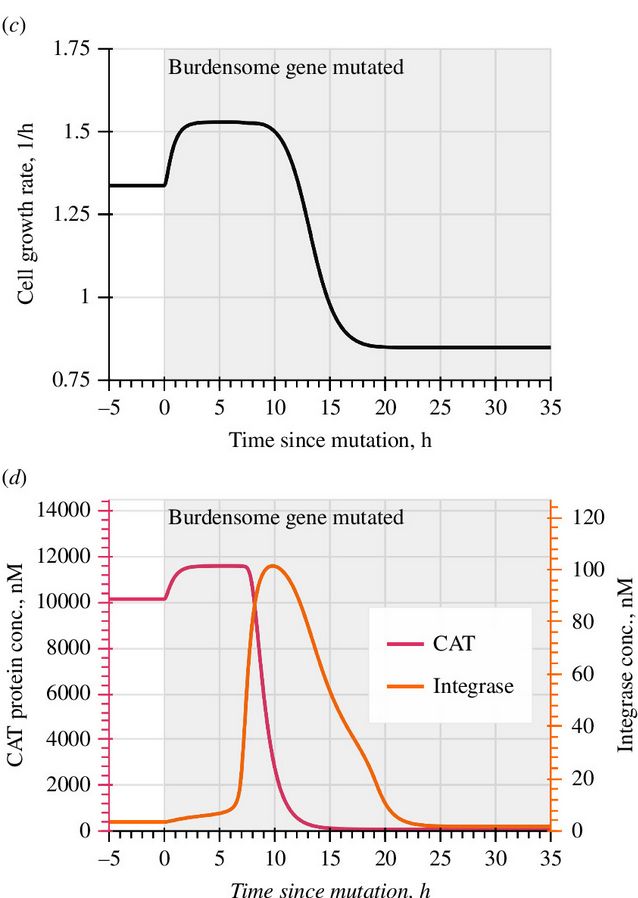
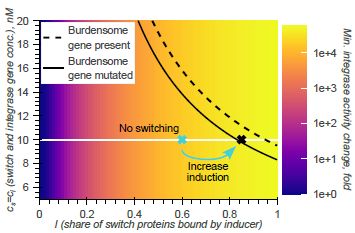
Model-guided gene circuit design for engineering genetically stable cell populations in diverse applications | Journal of The Royal Society Interface
Maintaining engineered cell populations’ genetic stability is a key challenge in synthetic
biology. Synthetic genetic constructs compete with a host cell’s native genes for
expression resources, burde...
doi.org
Kirill Sechkar
@ksechkar.bsky.social
· Feb 12
Kirill Sechkar
@ksechkar.bsky.social
· Feb 12
Kirill Sechkar
@ksechkar.bsky.social
· Feb 12