
Shelley Minteer
@minteerlab.bsky.social
160 followers
120 following
19 posts
Chemistry professor at Missouri S&T, electrochemist, editor-in-chief of the ACS Au journals
Posts
Media
Videos
Starter Packs
Shelley Minteer
@minteerlab.bsky.social
· Aug 27

Understanding Palladium Ion Induced Bioelectrocatalysis in Shewanella oneidensis MR-1 through Electrochemical and Genetic Interrogations
Efficient extracellular electron transfer (EET) is critical to harnessing bacteria like Shewanella oneidensis MR-1 in microbial electrochemical systems (MESs) aimed at sustainable energy production, environmental remediation, and resource recovery. This study investigates how palladium ions (Pd2+), which are of significant interest for catalysis and resource recovery, impact the bioelectrocatalysis of S. oneidensis MR-1 using electrochemical, transcriptomic, and microscopic methods. Pd2+ exposure significantly increased the observed current via EET, which correlated with substantially increased viable biofilm formation on the electrode. Transcriptomics revealed a coordinated cellular response, including upregulation of genes facilitating EET (the Mtr pathway), metabolism, motility, and biofilm processes, alongside downregulation of competing pathways. Cellular reduction of Pd2+ to palladium nanoparticles, mainly near the outer membrane, was confirmed via microscopy. Pd2+ boosts S. oneidensis MR-1 bioelectrocatalysis through the combined effects of promoting biofilm development and upregulating essential cellular pathways, informing fundamental strategies for enhanced MESs targeted for sustainable engineering, bioremediation, and resource and material recovery applications.
pubs.acs.org
Shelley Minteer
@minteerlab.bsky.social
· Aug 14

Missouri S&T awarded five-year $19.8 million grant to lead Center for Chemical Innovation
Missouri S&T has been awarded a $19.8 million collaborative agreement to renew the National Science Foundation’s Center for Synthetic Organic Electrochemistry. This chemical innovation center will be ...
news.mst.edu
Shelley Minteer
@minteerlab.bsky.social
· Jun 13
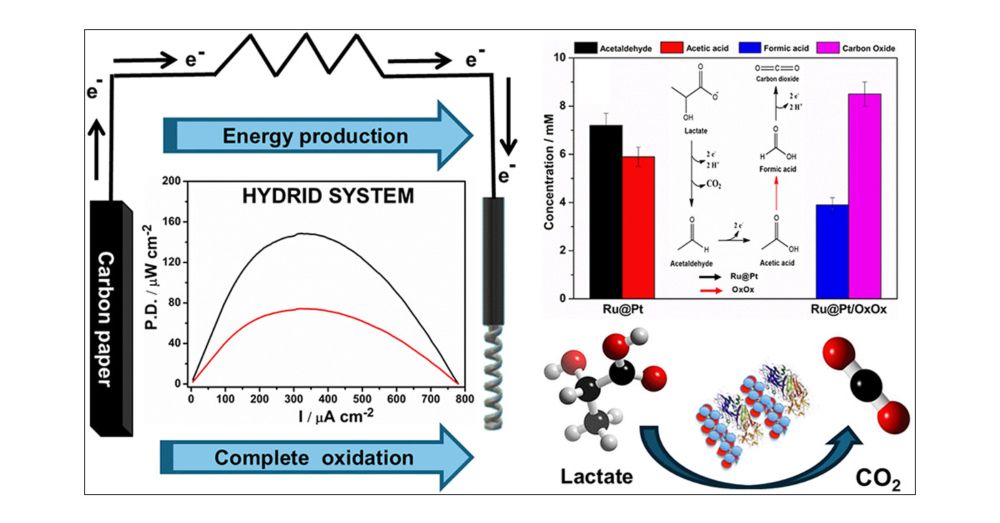
Enhanced Biofuel Cells Based on a Hybrid Enzymatic/Bimetallic Composite for Complete Lactate Catalytic Electrooxidation
We describe complete lactate electrooxidation in an enzymatic biofuel cell that combines the catalytic action of the bimetallic composite Ru@Pt-CNT and the enzyme oxalate oxidase (OxOx). The Ru@Pt-CNT...
pubs.acs.org
Shelley Minteer
@minteerlab.bsky.social
· Apr 11
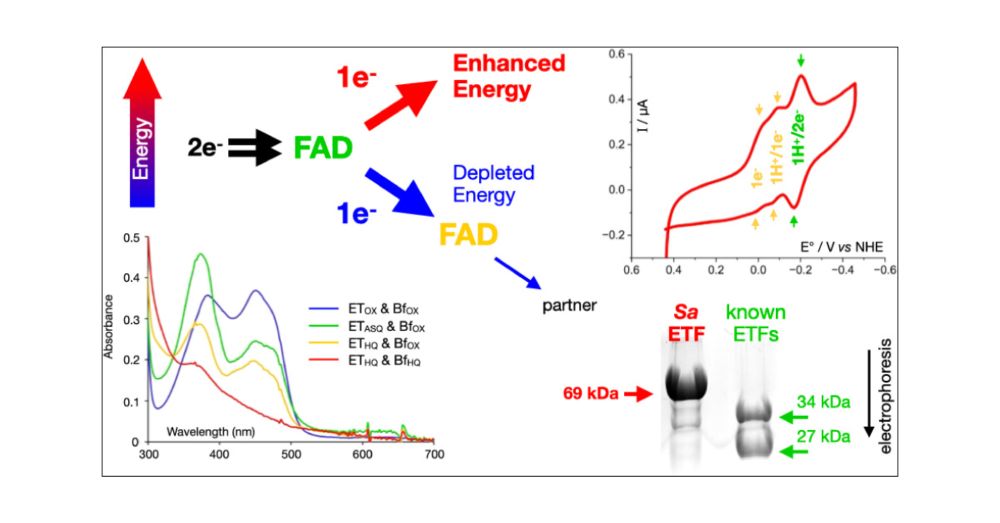
Electrochemical Observation and pH Dependence of All Three Expected Redox Couples in an Extremophilic Bifurcating Electron Transfer Flavoprotein with Fused Subunits
Bifurcating enzymes employ energy from a favorable electron transfer to drive unfavorable transfer of a second electron, thereby generating a more reactive product. They are therefore highly desirable in catalytic systems, for example, to drive challenging reactions such as nitrogen fixation. While most bifurcating enzymes contain air-sensitive metal centers, bifurcating electron transfer flavoproteins (bETFs) employ flavins. However, they have not been successfully deployed on electrodes. Herein, we demonstrate immobilization and expected thermodynamic reactivity of a bETF from a hyperthermophilic archaeon, Sulfolobus acidocaldarius (SaETF). SaETF differs from previously biochemically characterized bETFs in being a single protein, representing a concatenation of the two subunits of known ETFs. However, SaETF retains the chemical properties of heterodimeric bETFs, including possession of two FADs: one that undergoes sequential 1-electron (1e) reductions at high E° and forms an anionic semiquinone, and another that is amenable to lower-E° 2e reduction, including by NADH. We found homologous monomeric ETF genes in archaeal and bacterial genomes, accompanied by genes that also commonly flank heterodimeric ETFs, and SaETF’s sequence conservation is 50% higher with bETFs than with canonical ETFs. Thus, SaETF is best described as a bETF. Our direct electrochemical trials capture reversible redox couples for all three thermodynamically expected redox events. We document electrochemical activity over a range of pH values and reveal a conformational change coupled to proton acquisition that affects the electrochemical activity of the higher-E° FAD. Thus, this well-behaved monomeric bETF opens the door to bioinspired bifurcating devices or bifurcation on a chip.
pubs.acs.org
Shelley Minteer
@minteerlab.bsky.social
· Mar 18
Shelley Minteer
@minteerlab.bsky.social
· Mar 18
It is my great honor to receive the Pittcon Achievement Award! Thanks to all the current and former students for their hard work @waynestatechem.bsky.social @utahchemistry.bsky.social ! Also, thanks to
@minteerlab.bsky.social , TD, Siegi, and Lane @bakergrp.bsky.social for the continued support!
@minteerlab.bsky.social , TD, Siegi, and Lane @bakergrp.bsky.social for the continued support!

Shelley Minteer
@minteerlab.bsky.social
· Dec 23
Our Pick of the Week, out #OpenAccess in #ChemElectroChem as part of their Anniversary Special Collection by @minteerlab.bsky.social:
"Utility of Immobilized Metal Salens as Electrocatalysts: Fuel Cells and Organic Electrosynthesis"
bit.ly/CELC_2400445
#Chemsky #Chemistry
"Utility of Immobilized Metal Salens as Electrocatalysts: Fuel Cells and Organic Electrosynthesis"
bit.ly/CELC_2400445
#Chemsky #Chemistry

Reposted by Shelley Minteer
Prashant Kamat
@kamat.bsky.social
· Dec 10
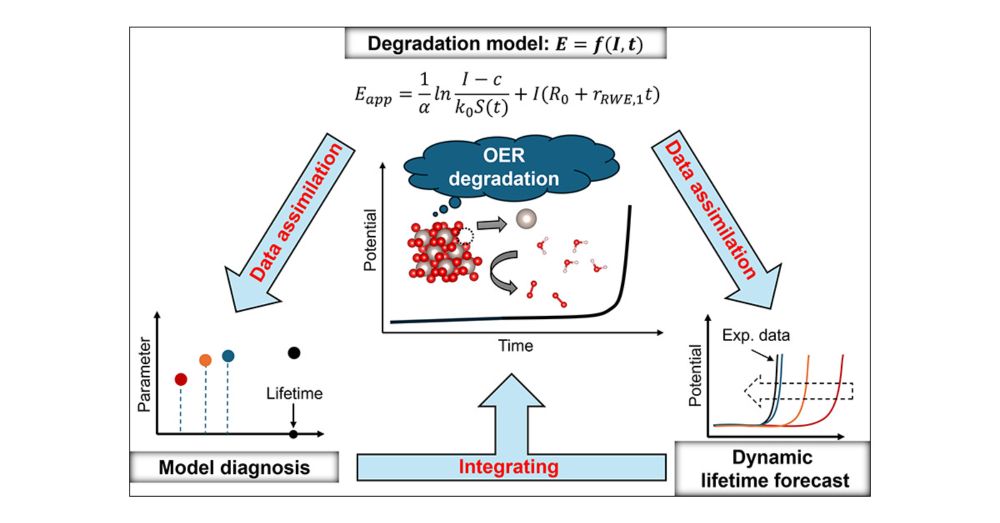
Accelerated Electrocatalyst Degradation Testing by Accurate and Robust Forecasting of Multidimensional Kinetic Model with Bayesian Data Assimilation
Degradation tests represent a significant bottleneck in electrochemical technology development, occasionally requiring tens of thousands of hours. Thus, reliable degradation forecasting in a short time frame is a game-changer in accelerating the establishment of future electrochemical devices. Herein, we show a multidimensional kinetic model for electrocatalyst degradation by quantifying the relationship among potential, current, and time, applicable under various conditions. Aiming to predict reliable degradation behaviors in shorter experimental timeframes and inspired by modern weather forecasting methods, we integrated Bayesian data assimilation with our model to expedite multidimensional parameter optimization. Consequently, we achieved accurate and robust forecasting of electrocatalyst lifetime by employing oxygen evolution reaction as a representative system: it takes just 300 h to obtain the final lifetime of close to 1000 h even with environmental noise. This data-driven approach can accelerate our understanding of the microscopic electrochemical mechanisms and simultaneously directly bridge this understanding to develop next-generation energy technologies.
pubs.acs.org
Reposted by Shelley Minteer
Shelley Minteer
@minteerlab.bsky.social
· Dec 10

Call For Nominations: ACS Au Journals 2025 Rising Stars | ACS Publications Chemistry Blog
These Special Issues will showcase original, cutting-edge research from across the chemical sciences, highlighting the early-career researchers conducting work at the forefront of these fields. Submit...
axial.acs.org
Reposted by Shelley Minteer
Introducing a new, automated approach for constructing Zone Diagrams in #Electrochemistry. Check out how it is possible to decipher complexity in electrochemical systems through the lens of geometry, out now in JACS! #ChemSky pubs.acs.org/doi/10.1021/...

A Geometric Interpretation of Kinetic Zone Diagrams in Electrochemistry
Electrochemical systems with increasing complexity are gaining importance in catalytic energy conversion applications. Due to the interplay between transport phenomena and chemical kinetics, predicting optimization is a challenge, with numerous parameters controlling the overall performance. Zone diagrams provide a way to identify specific kinetic regimes and track how variations in the governing parameters translate the system between either adverse or optimal kinetic states. However, the current procedures for constructing zone diagrams are restricted to simplified systems with a minimal number of governing parameters. We present a computationally based method that maps the entire parameter space of multidimensional electrochemical systems and automatically identifies kinetic regimes. Once the current output over a discrete set of parameters is interpreted as a geometric surface, its geometry encodes all of the information needed to construct a zone diagram. Zone boundaries and limiting zones are defined by curved and flat regions, respectively. This geometric framework enables a systematic exploration of the parameter space, which is not readily accessible by analytical or direct numerical methods. This will become increasingly valuable for the rational design of electrochemical systems with intrinsically high complexity.
pubs.acs.org










