w🌟 @tiansterzhangmd.bsky.social & me
...assuming EV+P 1L
✅FGFR3 alts: THOR/erda
🎯Trop-2: sacituzumab govitecan
🏁HER2 IHC 3+: T-DXd
Support by edu grants: Astellas, Gilead Sci, Merck, Seagen
#CME from @bonumce.bsky.social ℹ️👇
w🌟 @tiansterzhangmd.bsky.social & me
...assuming EV+P 1L
✅FGFR3 alts: THOR/erda
🎯Trop-2: sacituzumab govitecan
🏁HER2 IHC 3+: T-DXd
Support by edu grants: Astellas, Gilead Sci, Merck, Seagen
#CME from @bonumce.bsky.social ℹ️👇
@drchoueiri.bsky.social @pgrivasmdphd.bsky.social @davidaggen.bsky.social

@drchoueiri.bsky.social @pgrivasmdphd.bsky.social @davidaggen.bsky.social

@jrgralow.bsky.social
@elisabettabonzano.bsky.social
@stolaney1.bsky.social
@stoverlab.bsky.social
@pgrivasmdphd.bsky.social
@axelmerseburger.bsky.social
@drnataliagandur.bsky.social
@biagioricciutimd.bsky.social
@fernandoonco.bsky.social
@drchoueiri.bsky.social @pgrivasmdphd.bsky.social @davidaggen.bsky.social @erplimackmd.bsky.social

@drchoueiri.bsky.social @pgrivasmdphd.bsky.social @davidaggen.bsky.social @erplimackmd.bsky.social
#Oncology #BladderCancer

#Oncology #BladderCancer











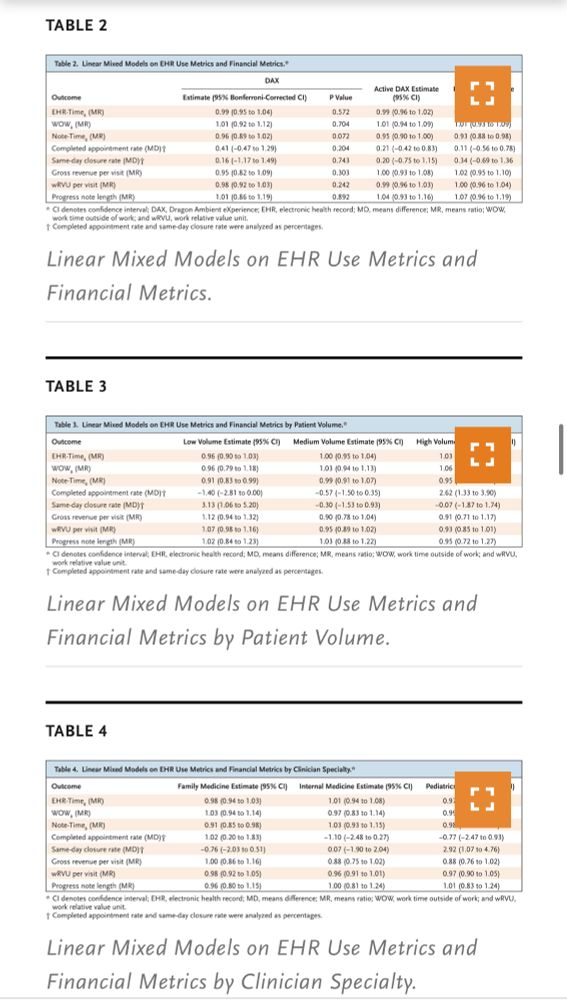
@NEJM study on @NuanceDragon DAX Copilot, an AI-powered documentation tool, found minimal impact on EHR efficiency & financial metrics❗️
💡AI tools need to be developed in comjuction with 🩺🥼🧑⚕️ not in silos
#AIinMedicine #LLMs @oncoalert.bsky.social
@NEJM study on @NuanceDragon DAX Copilot, an AI-powered documentation tool, found minimal impact on EHR efficiency & financial metrics❗️
💡AI tools need to be developed in comjuction with 🩺🥼🧑⚕️ not in silos
#AIinMedicine #LLMs @oncoalert.bsky.social

This question & the increase in #lungcancer diagnoses among younger women vs men are the genesis for the #HearHer campaign.
Read more & help us change this👇
healio.com/news/hematolog…#LCSMSM

This question & the increase in #lungcancer diagnoses among younger women vs men are the genesis for the #HearHer campaign.
Read more & help us change this👇
healio.com/news/hematolog…#LCSMSM
#Oncologists exposed to scenarios of a “nice” or “demanding” pt were associated with less recommendations of chemotherapy near the #EOL .
#heme/onc #lcsm #lcam #oncsky #medsky
doi.org/10.1093/onco...

#Oncologists exposed to scenarios of a “nice” or “demanding” pt were associated with less recommendations of chemotherapy near the #EOL .
#heme/onc #lcsm #lcam #oncsky #medsky
doi.org/10.1093/onco...
•The #HearHer campaign focuses on gender disparities in lung cancer diagnosis and clinical trial enrollment.
•Clinicians should take women’s concerns seriously and make use of imaging for early detection.
Article here: www.healio.com/news/hematol...

•The #HearHer campaign focuses on gender disparities in lung cancer diagnosis and clinical trial enrollment.
•Clinicians should take women’s concerns seriously and make use of imaging for early detection.
Article here: www.healio.com/news/hematol...


#MedSky #IDSky #Pediatrics #GetVaccinated
#MedSky #IDSky #Pediatrics #GetVaccinated

#PROSCA24 #BLADDR24 & #RENALC24
FINAL STATS with OVER 21 million impression on X
SHOUT OUT TO Our Ambassadors Specially
@drnataliagandur.bsky.social
@barbaramelao.bsky.social
@pgrivasmdphd.bsky.social
@achoud72.bsky.social

#PROSCA24 #BLADDR24 & #RENALC24
FINAL STATS with OVER 21 million impression on X
SHOUT OUT TO Our Ambassadors Specially
@drnataliagandur.bsky.social
@barbaramelao.bsky.social
@pgrivasmdphd.bsky.social
@achoud72.bsky.social
society.asco.org/about-asco/a...
society.asco.org/about-asco/a...



