
With the beginning of the new year, the Cluster of Excellence RESOLV has officially started its third funding period (2026 to 2032) with new Research Areas.
Learn more about our new Research Areas: www.solvation.de/research
#SolvationScience

With the beginning of the new year, the Cluster of Excellence RESOLV has officially started its third funding period (2026 to 2032) with new Research Areas.
Learn more about our new Research Areas: www.solvation.de/research
#SolvationScience



PhD Position 1 (application deadline Jan 5th):
www.uni-due.de/karriere/ste...
PhD Position 2 (application deadline Jan 22nd):
www.uni-due.de/karriere/ste...
Apply with cover letter and CV!
Re-posts appreciated!
PhD Position 1 (application deadline Jan 5th):
www.uni-due.de/karriere/ste...
PhD Position 2 (application deadline Jan 22nd):
www.uni-due.de/karriere/ste...
Apply with cover letter and CV!
Re-posts appreciated!



See: pubs.rsc.org/en/Content/A...
See: pubs.rsc.org/en/Content/A...

Politicians actually trying to solve problems can achieve a lot!
www.washingtonpost.com/climate-solu...

Politicians actually trying to solve problems can achieve a lot!
www.washingtonpost.com/climate-solu...




We've investigated the high pressure behavior of halogen bonding and found some unexpected solvent @solvationsci.bsky.social dependence:
pubs.acs.org/doi/10.1021/...
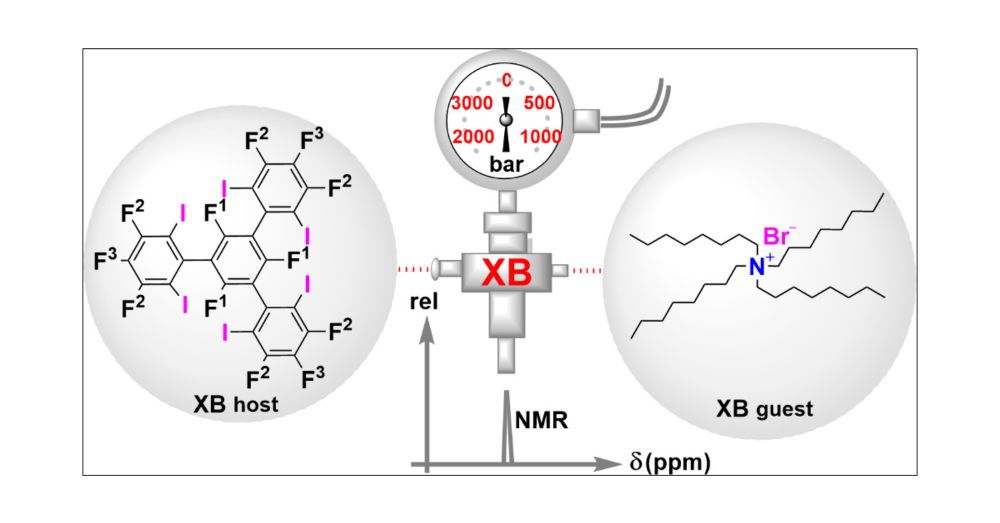
We've investigated the high pressure behavior of halogen bonding and found some unexpected solvent @solvationsci.bsky.social dependence:
pubs.acs.org/doi/10.1021/...


Die Klimakrise und weitere Umweltkrisen (Biodiversitätsverlust, Überlastung biogeochemischer Stoffkreisläufe, ...) sind mittelfristig die größte Bedrohung für Sicherheit, Wirtschaft und Wohlstand, Demokratie, Zivilisation und Menschenleben.
➡️

Die Klimakrise und weitere Umweltkrisen (Biodiversitätsverlust, Überlastung biogeochemischer Stoffkreisläufe, ...) sind mittelfristig die größte Bedrohung für Sicherheit, Wirtschaft und Wohlstand, Demokratie, Zivilisation und Menschenleben.
➡️


PS: For all biochemists and biologists who have started to follow us: thank you for your interest, but be aware that we don't really work much in these areas...
PS: For all biochemists and biologists who have started to follow us: thank you for your interest, but be aware that we don't really work much in these areas...

