www.nature.com/articles/s41...

www.nature.com/articles/s41...


Unravelling UV–vis spectra of U4+-containing aqueous solutions and their relationship with the nature of complexes present at different pH levels. Selected as Featured article in Inorg. Chem. and a cornerstone of
@gemarh.bsky.social PhD at @unisevilla.bsky.social

Unravelling UV–vis spectra of U4+-containing aqueous solutions and their relationship with the nature of complexes present at different pH levels. Selected as Featured article in Inorg. Chem. and a cornerstone of
@gemarh.bsky.social PhD at @unisevilla.bsky.social

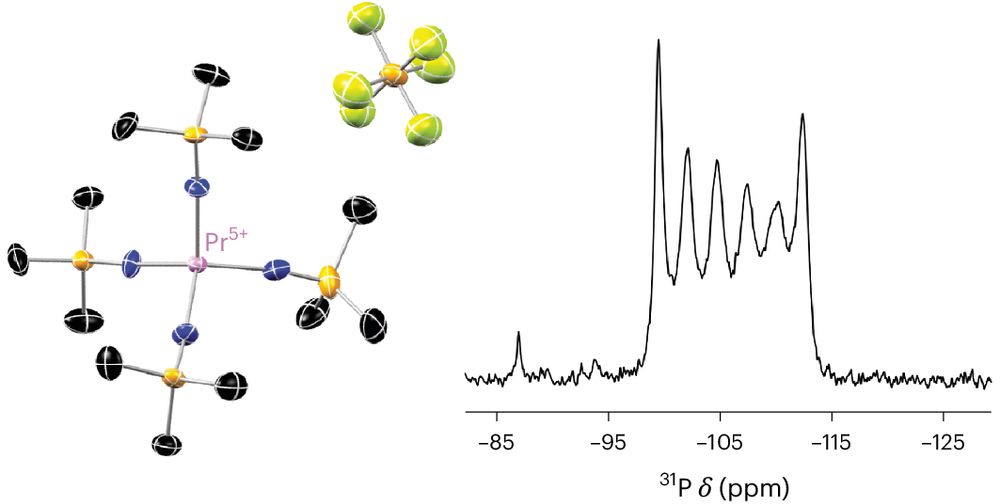
www.chemistryworld.com/news/super-s...
www.chemistryworld.com/news/super-s...
pubs.rsc.org/en/content/a...

pubs.rsc.org/en/content/a...
Read it for free 👉pubs.rsc.org/doi/D4S...
#ChemSky

Read it for free 👉pubs.rsc.org/doi/D4S...
#ChemSky
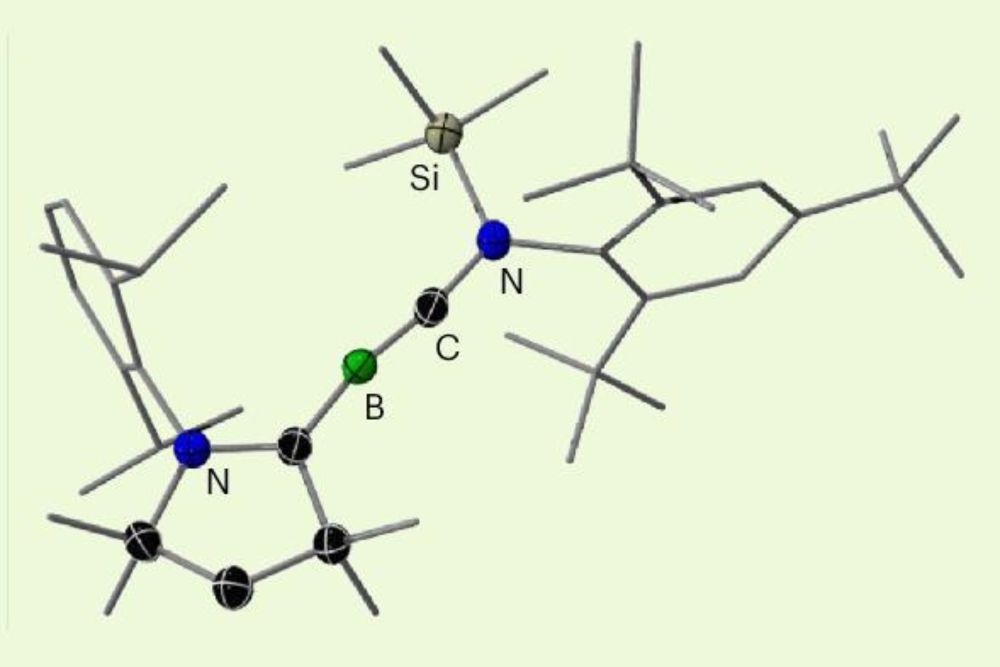





#NationalPeriodicTableDay #chemsky🧪 #scinews

#NationalPeriodicTableDay #chemsky🧪 #scinews