Utah Chemistry
@utahchemistry.bsky.social
52 followers
37 following
45 posts
University of Utah Department of Chemistry
https://linktr.ee/uofu_chemistry
Posts
Media
Videos
Starter Packs
Reposted by Utah Chemistry
Reposted by Utah Chemistry
Reposted by Utah Chemistry
Reposted by Utah Chemistry
Utah Chemistry
@utahchemistry.bsky.social
· Aug 21

Chemistry Department Hosts First Utah Electrochemistry Symposium - Department of Chemistry
Profs. Henry White, Shelley Minteer, and Long Luo hosted the first Utah Electrochemistry Symposium (UTES) on July 25–26, 2025, at the University of Utah’s Department...
www.chemistry.utah.edu
Reposted by Utah Chemistry
Jamie Cadge
@jamiecadge.bsky.social
· Aug 15

A Data Science-Guided Approach for the Development of Nickel-Catalyzed Homo-Diels–Alder Reactions
The Ni-catalyzed homo-Diels–Alder (hDA) reaction represents a convergent but under-investigated approach to preparing bridged bicyclic ring systems. Using the kraken monophosphine descriptor library, ...
doi.org
Utah Chemistry
@utahchemistry.bsky.social
· Jul 22

Dr. Sanaz Habibi Awarded Stockham Medal - Department of Chemistry
Congratulations to Dr. Sanaz Habibi, recent Chemistry PhD graduate, on being awarded the 2025 Thomas G. Stockham Medal for Conspicuously Effective Teaching! The Stockham Medal...
www.chemistry.utah.edu
Reposted by Utah Chemistry
Luo Lab
@luolab-utah.bsky.social
· Jul 21
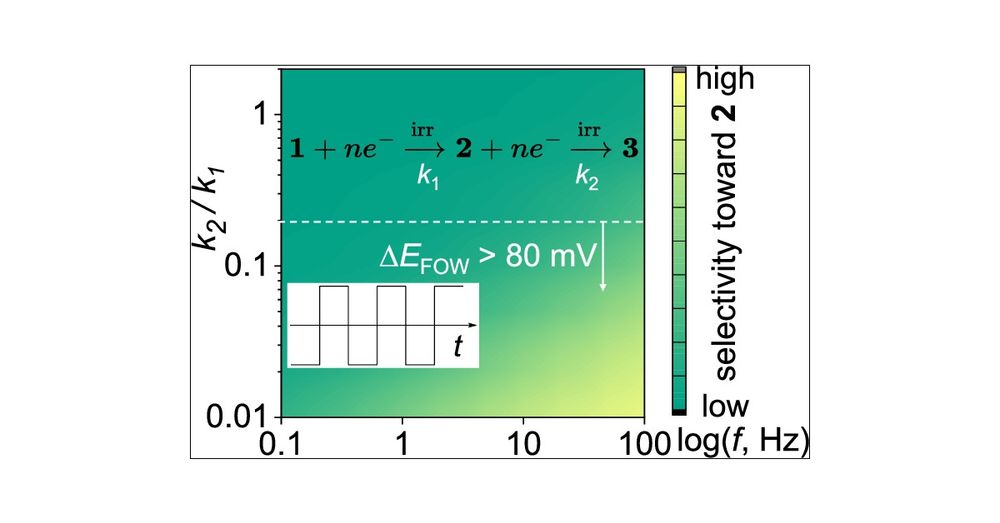
Origin of Selectivity in Alternating Current-Enabled Partial Reduction of (Hetero)Arenes: A Case Study of Two Consecutive Irreversible Electrochemical Steps
Herein, we investigate the origin of selectivity in the alternating current (AC)-enabled partial reduction of (hetero)arenes to cyclic alkenes. Reduction of (hetero)arenes can be considered as a reaction involving two consecutive irreversible electrochemical steps: the first generates the desired cyclic alkene, while the second leads to its undesired overreduction. Conventional constant current or voltage (DC) electrolysis results in poor selectivity toward the partial reduction products, originating from overreduction and base-induced decomposition of the desired product. Fast-scan cyclic voltammetry shows that the rate constant for the first reduction (k1) exceeds that of the second one (k2). Finite element simulations based on this experimental finding semiquantitatively capture the frequency-dependent selectivity observed in AC electrolysis experiments (i.e., increasing the AC frequency enhances selectivity). The results further reveal that AC electrolysis mitigates the low selectivity by only collecting the products at the initial stage of the reduction reaction, which is mostly under a kinetically controlled regime. We then extend the finite element model and introduce ΔEFOW, the foot-of-the-wave potential difference between cyclic voltammograms of substrate and partial reduction product, as an accessible proxy for k2/k1. A ΔEFOW > 80 mV predicts synthetically useful selectivity (>30%) toward the partial reduction product below 100 Hz.
pubs.acs.org
Utah Chemistry
@utahchemistry.bsky.social
· Jul 21
Utah Chemistry
@utahchemistry.bsky.social
· Jul 18

Aurora Clark Named New Chair of Chemistry - Department of Chemistry
The University of Utah Department of Chemistry welcomes Aurora Clark as its new chair beginning July 1, replacing interim chair Peter Armentrout. She brings with...
chemistry.utah.edu
Reposted by Utah Chemistry






















