Aaron Kesselheim
@akesselheim.bsky.social
590 followers
66 following
62 posts
Professor of Medicine, Brigham and Women's Hospital/Harvard Medical School; Director, Program On Regulation, Therapeutics, And Law (PORTAL)
Posts
Media
Videos
Starter Packs
Reposted by Aaron Kesselheim
Reposted by Aaron Kesselheim
Yale CRRIT
@yalecrrit.bsky.social
· Jul 21
Aaron Kesselheim
@akesselheim.bsky.social
· Jul 13
Aaron Kesselheim
@akesselheim.bsky.social
· Jun 28
Congress Should Remove The Rare Disease Carve-Out From Medicare Drug Price Negotiation, Not Expand It | Health Affairs Forefront
Expansion of the Medicare drug price negotiation rare disease drug carve-out would unnecessarily limit the number of drugs eligible for negotiation, allow for continued high drug prices for products that earn billions of dollars in Medicare, and introduce a new set of misaligned incentives.
www.healthaffairs.org
Aaron Kesselheim
@akesselheim.bsky.social
· Jun 26
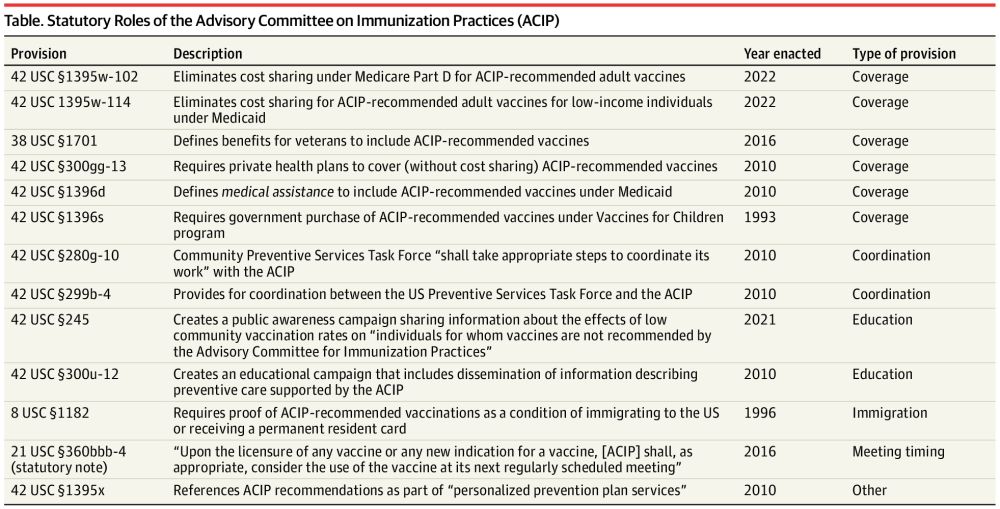
Legal Challenges for the Advisory Committee on Immunization Practices
This Perspective offers an analysis of the legal foundations in the context of these and other challenges to the role of the Advisory Committee on Immunization Practices.
jamanetwork.com
Aaron Kesselheim
@akesselheim.bsky.social
· May 13
Aaron Kesselheim
@akesselheim.bsky.social
· Feb 16

Spending After Sacubitril-Valsartan vs Renin-Angiotensin System Blockers for Heart Failure
This cohort study assesses health care costs after initiating sacubitril-valsartan compared to an angiotensin-converting enzyme inhibitor or an angiotensin II receptor-blocker in Medicare patients bei...
jamanetwork.com
Aaron Kesselheim
@akesselheim.bsky.social
· Feb 16

Out of Pocket Getting Out of Hand — Reducing the Financial Toxicity of Rapidly Approved Drugs | NEJM
The FDA often exercises flexibility in deciding whether to approve highly promising
drugs for patients in desperate need of treatment options. But it doesn’t consider
a drug’s likely financial toxi...
www.nejm.org
Aaron Kesselheim
@akesselheim.bsky.social
· Feb 14

Predicting patent challenges for small-molecule drugs: A cross-sectional study
Ally Memedovich and colleagues report on patent challenges within the first year of eligibility among small-molecule drugs approved by the FDA from 2007-2018 and investigate the extent to which market...
journals.plos.org
Reposted by Aaron Kesselheim












