Andreu Tortajada Navarro
@antorna.bsky.social
310 followers
300 following
15 posts
Ambizione Fellow/Junior Group Leader (University of Fribourg 🇨🇭)
Organometallic chemistry and catalysis, playing with organosodium compounds and more 😁
He/him 🏳️🌈
www.andreutortajada.com
Posts
Media
Videos
Starter Packs
Reposted by Andreu Tortajada Navarro
Reposted by Andreu Tortajada Navarro
Reposted by Andreu Tortajada Navarro
Reposted by Andreu Tortajada Navarro
Reposted by Andreu Tortajada Navarro
Reposted by Andreu Tortajada Navarro
Reposted by Andreu Tortajada Navarro
Reposted by Andreu Tortajada Navarro
Nando López 🏳️🌈
@nandolopez.bsky.social
· Jul 16
Reposted by Andreu Tortajada Navarro
Reposted by Andreu Tortajada Navarro
Reposted by Andreu Tortajada Navarro
Andryj Borys
@andryjborys.bsky.social
· Apr 12

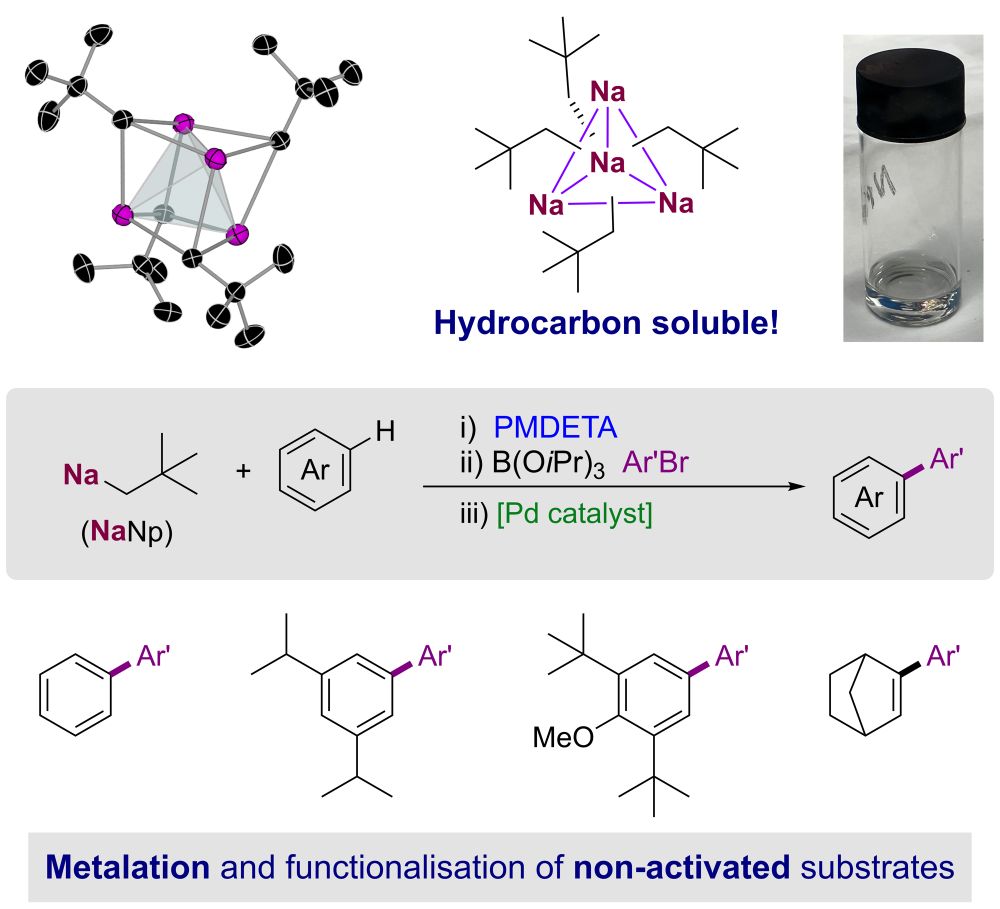
Magnesium Nickelate Complexes and Their Implications in Ni-Catalyzed Cross-Couplings of Aryl Fluorides and Aryl Ethers with Grignard Reagents
The nickel-catalyzed Kumada–Tamao–Corriu cross-coupling reaction is widely used to form C–C bonds and receives continued interest due to the unique ability of nickel to activate challenging organic electrophiles containing C–F and C–O bonds. Recent studies on the nickel-catalyzed cross-coupling of Ar–F and Ar–OMe electrophiles with organolithium nucleophiles have unveiled the key involvement of highly reactive anionic nickelates, in which Li and Ni cooperatively promote the activation of these substrates. However, the possible formation of related heterobimetallic intermediates when employing organomagnesium nucleophiles as cross-coupling partners still remains widely underexplored. Filling this gap in the knowledge, we use air-stable Ni-tris(olefin), Ni(4-Me-stb)3 (where stb = stilbene), as a Ni(0) precursor to systematically investigate its reactivity toward several organomagnesium and organolithium reagents, which has allowed for the structural and spectroscopic characterization of a new family of nickelate complexes. Their possible implications and the influence of their constitution and speciation on the outcome of Kumada–Tamao–Corriu cross-couplings are also investigated through a series of stoichiometric and catalytic reactions, which have uncovered a dramatic solvent effect, hinting at the formation of contacted ion pair species as key to maximizing Mg (or Li)/Ni(0) cooperativity.
pubs.acs.org
Reposted by Andreu Tortajada Navarro
Nature Synthesis
@natsynth.nature.com
· Mar 25

Iron-catalysed direct coupling of organosodium compounds - Nature Synthesis
Despite the abundance and non-toxic nature of sodium, organosodium reagents have rarely been used in organic synthesis. Now iron-catalysed homocoupling and C(sp2)–C(sp3) cross-coupling reactions of ar...
www.nature.com
Reposted by Andreu Tortajada Navarro
Reposted by Andreu Tortajada Navarro
Universität Bern
@unibe.ch
· Mar 25
Sustainable chemistry: producing molecules more environmentally friendly
Researchers from the University of Bern and the RIKEN research institute in Japan have made a significant advance in sustainable chemistry. They have succeeded in producing organic molecules through a...
mediarelations.unibe.ch
Reposted by Andreu Tortajada Navarro