Steve Diggle
@digglelab.bsky.social
2.8K followers
1.1K following
240 posts
Professor of Microbiology at Georgia Tech. Interested in bacterial interactions, quorum sensing, biofilms, antimicrobial resistance and bacteriocins (R-pyocins). www.thedigglelab.com.
Posts
Media
Videos
Starter Packs
Pinned
Steve Diggle
@digglelab.bsky.social
· May 29
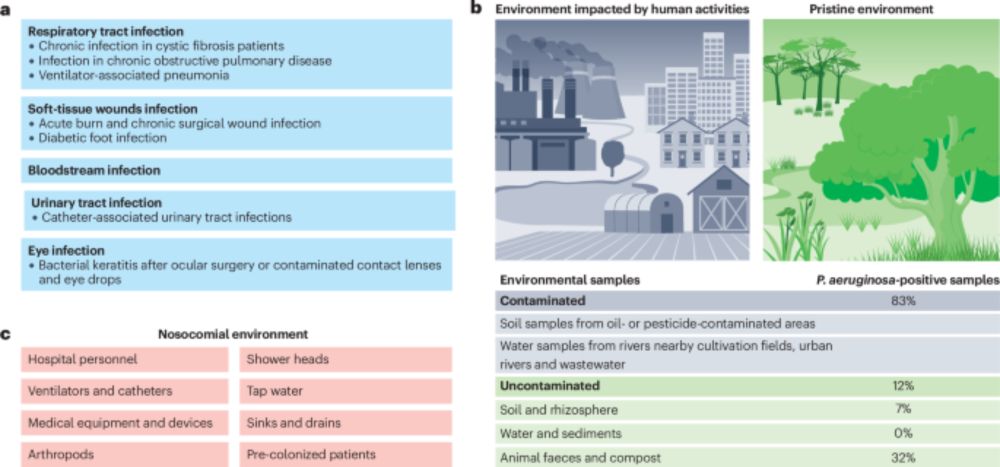
Pseudomonas aeruginosa: ecology, evolution, pathogenesis and antimicrobial susceptibility - Nature Reviews Microbiology
Pseudomonas aeruginosa is a functionally versatile bacterium, a leading opportunistic human pathogen and a model organism in microbiology. In this Review, Letizia, Diggle and Whiteley discuss P. aerug...
www.nature.com
Reposted by Steve Diggle
Reposted by Steve Diggle
Reposted by Steve Diggle
Reposted by Steve Diggle
Reposted by Steve Diggle
Reposted by Steve Diggle
Steve Diggle
@digglelab.bsky.social
· Sep 4
Steve Diggle
@digglelab.bsky.social
· Sep 3
Reposted by Steve Diggle
Alan McNally
@alanmcn1.bsky.social
· Sep 3

Assistant Professor (Research and Education) in Bacterial Genomics at University of Birmingham
Apply now for the Assistant Professor (Research and Education) in Bacterial Genomics role on jobs.ac.uk - the leading job board for higher education jobs. View details.
www.jobs.ac.uk
Steve Diggle
@digglelab.bsky.social
· Aug 28
Steve Diggle
@digglelab.bsky.social
· Aug 25

Host niche-specific challenges hindering the treatment of polymicrobial infections | Journal of Bacteriology
Antimicrobial recalcitrance in pathogenic microbes is a growing problem around the world (1–3). In addition to struggling to keep up with the growing multitude of organisms evolving resistance mechanisms, we must also contend with a variety of other issues that hinder antibiotic effectiveness. For example, polymicrobial infections are now understood to be a major problem in the world of antimicrobial recalcitrance due to the ability of certain microbial communities to work together via polymicrobial synergism. In fact, many infections that are recalcitrant to antimicrobials have been determined to be polymicrobial (4). Polymicrobial infections have been shown to significantly increase the cost of patient treatment and hospital length of stay, costing thousands of dollars for the individual (5). Polymicrobial chronic wounds alone now cost more than 28 billion dollars a year and affect more than five to seven million Americans every year (6, 7). In addition to battling complex communities of microorganisms, we must also determine the best way to prescribe the same antibiotics across multiple body sites with minimal impact on patients. Antibiotic prescription can have negative impacts at sites across the body, including allergic reactions and inhibiting or killing the host microbiome (8, 9). As every patient is unique (different immune responses, hormone levels, and ages), and each body site is different (nutrient composition and stressors), we must adapt our antibiotic prescription methods to most effectively treat the individual and their illness. This minireview focuses on the challenges that hinder effective antimicrobial prescription across the body, emphasizing the importance of accounting for distinct polymicrobial communities and niche-specific stressors to the bacteria that differ at each body site.
journals.asm.org
Steve Diggle
@digglelab.bsky.social
· Aug 18
Reposted by Steve Diggle
Steve Diggle
@digglelab.bsky.social
· Aug 6
Steve Diggle
@digglelab.bsky.social
· Aug 6