Emily Prevost
@emilyprevost.bsky.social
67 followers
85 following
24 posts
Behavioral Neuroscience PhD candidate in the Root Lab at the University of Colorado Boulder. Cellular heterogeneity in motivational systems. Expected graduation spring 2026. Gardener, skier, dog mom, and backyard chicken farmer. she/her
Posts
Media
Videos
Starter Packs
Emily Prevost
@emilyprevost.bsky.social
· Feb 27
Emily Prevost
@emilyprevost.bsky.social
· Feb 27
Emily Prevost
@emilyprevost.bsky.social
· Feb 27
Emily Prevost
@emilyprevost.bsky.social
· Feb 27
Emily Prevost
@emilyprevost.bsky.social
· Feb 27
Emily Prevost
@emilyprevost.bsky.social
· Feb 27
Emily Prevost
@emilyprevost.bsky.social
· Feb 27
Emily Prevost
@emilyprevost.bsky.social
· Feb 27
Emily Prevost
@emilyprevost.bsky.social
· Feb 27

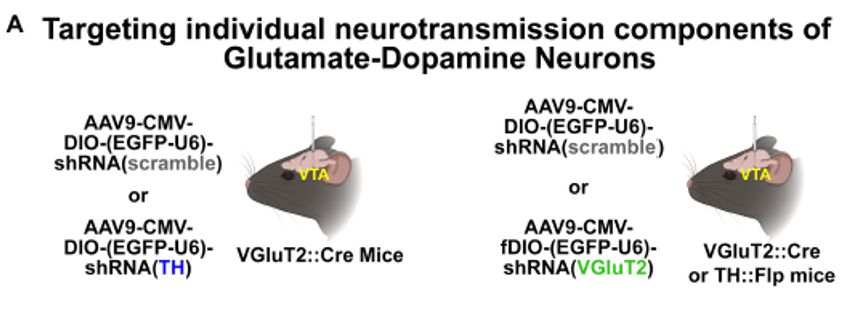
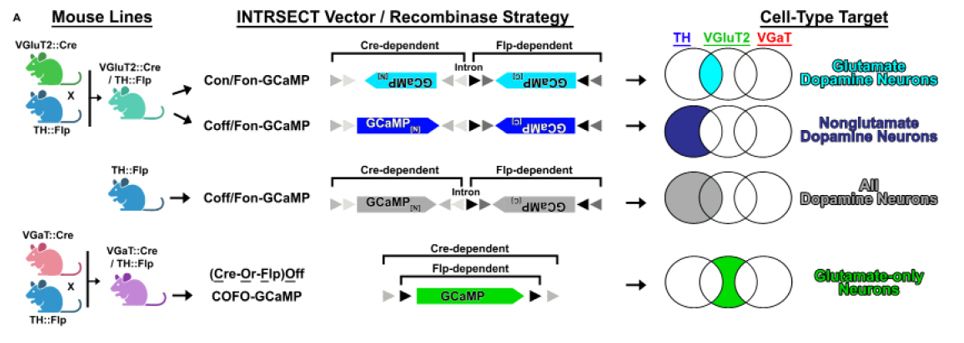
Untangling dopamine and glutamate in the ventral tegmental area
Ventral tegmental area (VTA) dopamine neurons are of great interest for their central roles in motivation, learning, and psychiatric disorders. While hypotheses of VTA dopamine neuron function posit a...
www.biorxiv.org
Reposted by Emily Prevost