Eric Yang
@ericyangchem.bsky.social
130 followers
120 following
7 posts
Inorganic chemist| RCSA Fellow @rescorp.org and postdoc with the Figueroa Group @ucsd.bsky.social | PhD with @goicoecheagroup.bsky.social @oxfordchemistry.bsky.social | (he/him)
Posts
Media
Videos
Starter Packs
Eric Yang
@ericyangchem.bsky.social
· Jun 27
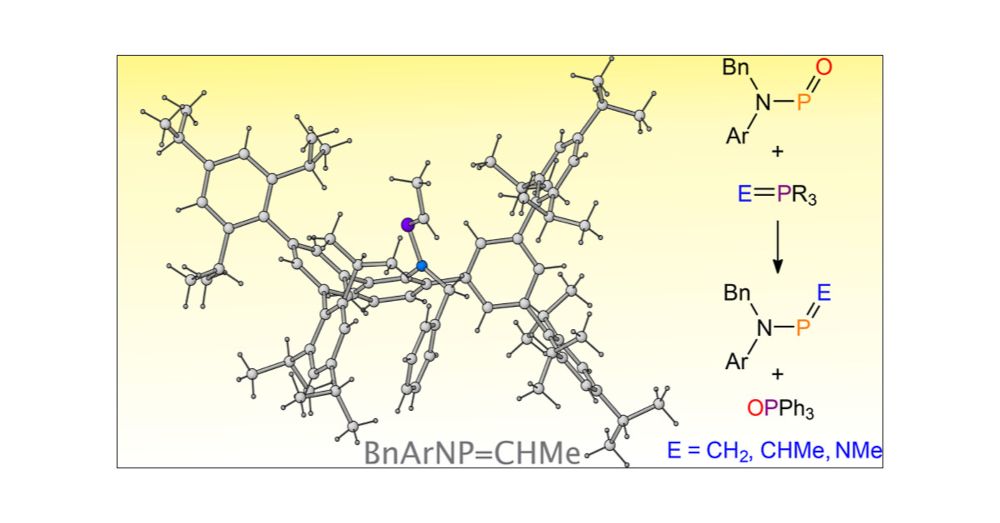
Eric Yang
@ericyangchem.bsky.social
· Jun 18
Reposted by Eric Yang
Eric Yang
@ericyangchem.bsky.social
· Apr 15
Eric Yang
@ericyangchem.bsky.social
· Apr 12
Reposted by Eric Yang
ChemComm
@chemcomm.rsc.org
· Mar 3
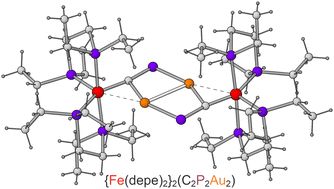
Cyaphide group transfer from covalent metal complexes: contrasting pathways to transmetallation
We describe two contrasting transmetallation reactions between the gold(i) cyaphide complex, Au(IDipp)(CP) (IDipp = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene), and low oxidation state main-group and transition-metal complexes. The reactivity observed highlights the pseudo-halide character of the cyaph
pubs.rsc.org
Reposted by Eric Yang
Eric Yang
@ericyangchem.bsky.social
· Dec 11