EuroMOF
@euromof.bsky.social
220 followers
270 following
25 posts
Leading biannual events, uniting experts from academia and industry to discuss advancements in Metal-Organic Frameworks (MOFs) and Porous Polymers. Initiated by DECHEMA, they serve as a platform for innovation, collaboration & knowledge exchange
Posts
Media
Videos
Starter Packs
Pinned
Reposted by EuroMOF
Reposted by EuroMOF
Reposted by EuroMOF
Reposted by EuroMOF
Reposted by EuroMOF
EuroMOF
@euromof.bsky.social
· 16d
Reposted by EuroMOF
EuroMOF
@euromof.bsky.social
· 22d

6th European Conference on Metal Organic Frameworks and Porous Polymers (EuroMOF2025)
Welcome
Dear Ladies and Gentlemen,
We are delighted to welcome you to the 6th European Conference on Metal Organic Frameworks and Porous Polymers (EuroMOF2025).
Building on the success of previou...
www.euromof2025.com
Reposted by EuroMOF
EuroMOF
@euromof.bsky.social
· Jun 11

Fraunhofer Research Award for TUD chemist Prof. Stefan Kaskel
Prof… | Technische Universität Dresden
Fraunhofer Research Award for TUD chemist Prof. Stefan Kaskel
Prof. Kaskel Stefan, Professor of Inorganic Chemistry at TUD School of Science, together with Dr. Benjamin Schumm and Dr. Holger Althues ...
www.linkedin.com
Reposted by EuroMOF
Reposted by EuroMOF
Reposted by EuroMOF
FAST Group
@fast-group.bsky.social
· Apr 29
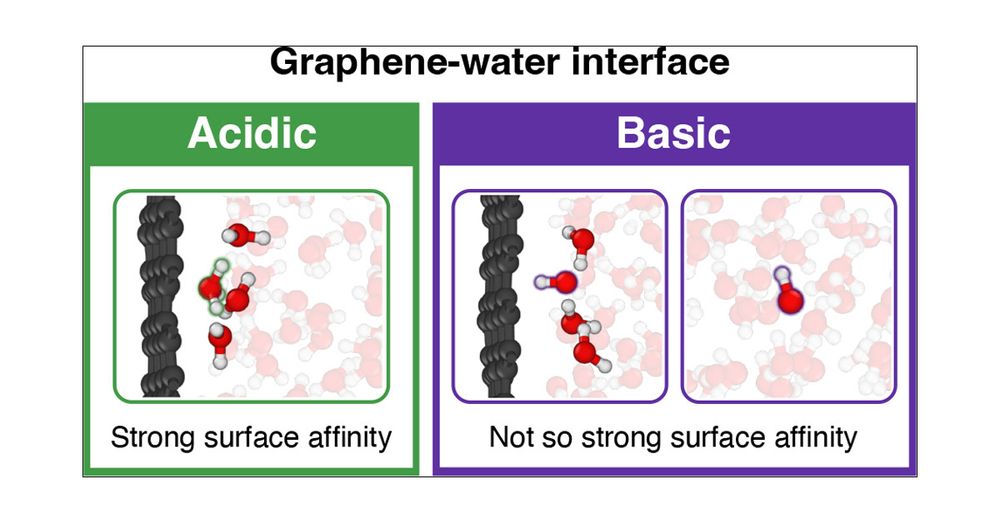
Protons Accumulate at the Graphene–Water Interface
Water’s ability to autoionize into hydroxide and hydronium ions profoundly influences surface properties, rendering interfaces either basic or acidic. While it is well-established that protons show an affinity to the air–water interface, a critical knowledge gap exists in technologically relevant surfaces like the graphene–water interface. Here we use machine learning-based simulations with first-principles accuracy to unravel the behavior of hydroxide and hydronium ions at the graphene–water interface. Our findings reveal that protons accumulate at the graphene–water interface, with the hydronium ion predominantly residing in the first contact layer of water. In contrast, the hydroxide ion exhibits a bimodal distribution, found both near the surface and further away from it. Analysis of the underlying electronic structure reveals local polarization effects, resulting in counterintuitive charge rearrangement. Proton propensity to the graphene–water interface challenges the interpretation of surface experiments and is expected to have far-reaching consequences for ion conductivity, interfacial reactivity, and proton-mediated processes.
doi.org
Reposted by EuroMOF
Randall Snurr
@randallsnurr.bsky.social
· May 10

Experimental and theoretical investigation of hydrogen sorption by SnO2 nanostructures in a metal–organic framework scaffold
SnO2 nanostructures decorated with Pd clusters were installed in the porous metal–organic framework (MOF) material NU-1000 and investigated as hydrogen storage materials. The proposed concept is to...
www.tandfonline.com






















