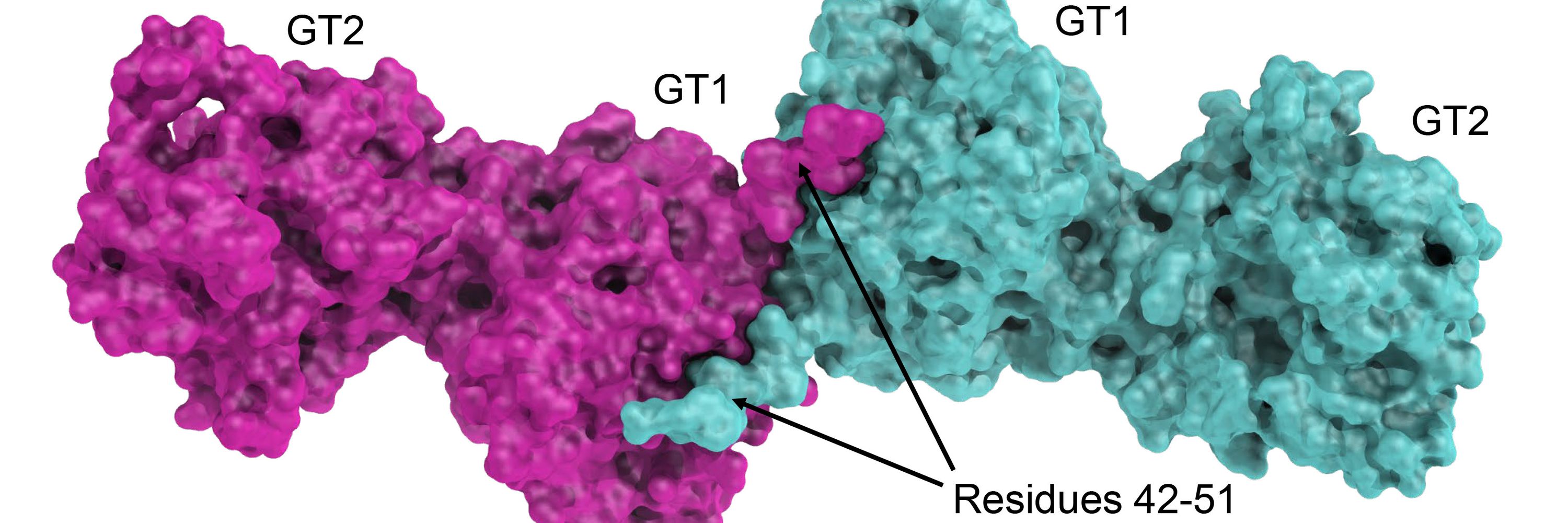
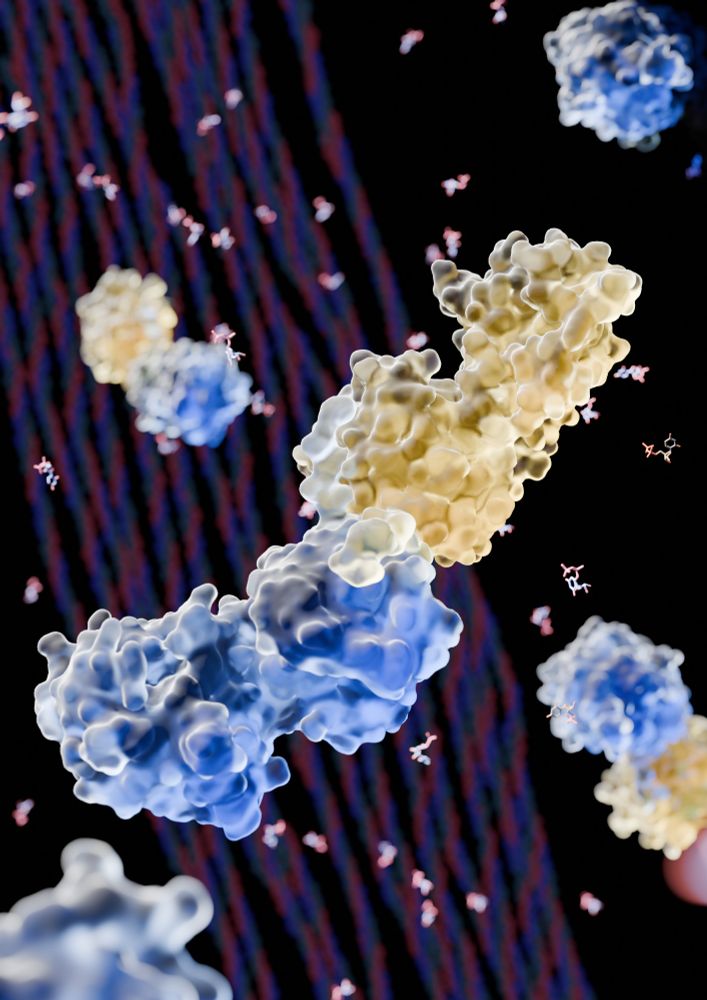
Forneris Lab @UniPV
@fornerislab.bsky.social
170 followers
320 following
230 posts
The Armenise Harvard laboratory of Structural Biology at the University of Pavia, Italy. We study molecular recognition using #structuralbiology (#crystallography and #cryoEM).
http://fornerislab.unipv.it
Posts
Media
Videos
Starter Packs
Reposted by Forneris Lab @UniPV
Reposted by Forneris Lab @UniPV