Gilliard Group
@gilliardgroup.bsky.social
490 followers
32 following
16 posts
Main-Group | Organometallic Chemistry | Luminescent Materials Research Group @MITofficial.bsky.social led by @rjgilliard.bsky.social Website: https://gilliardlab.mit.edu *Account managed by members of the group.
Posts
Media
Videos
Starter Packs
Reposted by Gilliard Group
Reposted by Gilliard Group
Reposted by Gilliard Group
Reposted by Gilliard Group
Reposted by Gilliard Group
Gilliard Group
@gilliardgroup.bsky.social
· Jun 11
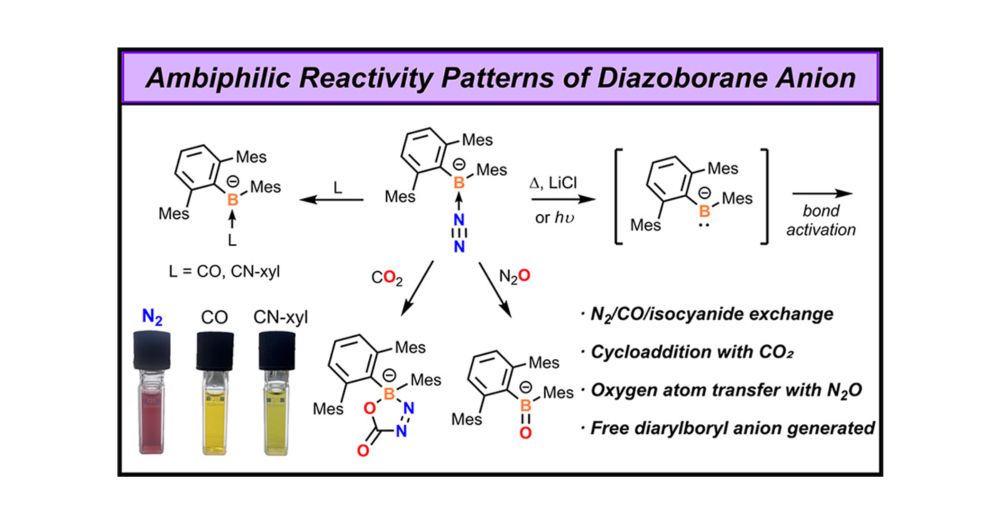
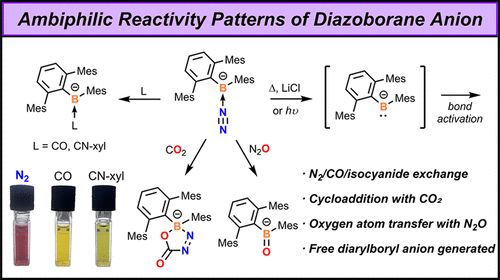
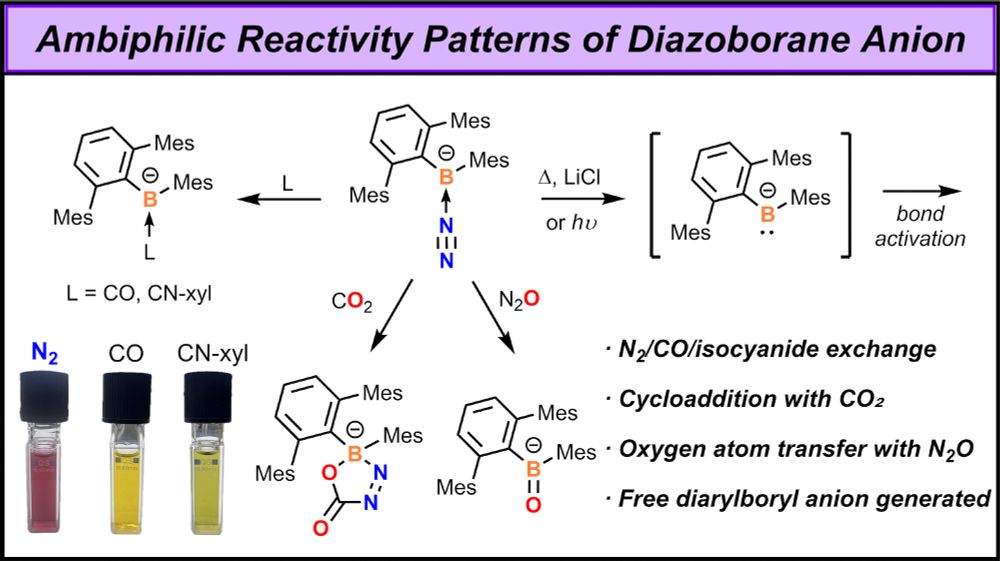
Unlocking the Ambiphilicity of the Boryl Anion: Synthesis and Reactivity of an Anionic Diazoborane
Reported boryl anions (R2B–) often exhibit n–p conjugation between the boron atom and adjacent heteroatoms, with their nucleophilicity being the primary focus. In this work, we present evidence that a...
pubs.acs.org
Gilliard Group
@gilliardgroup.bsky.social
· May 23
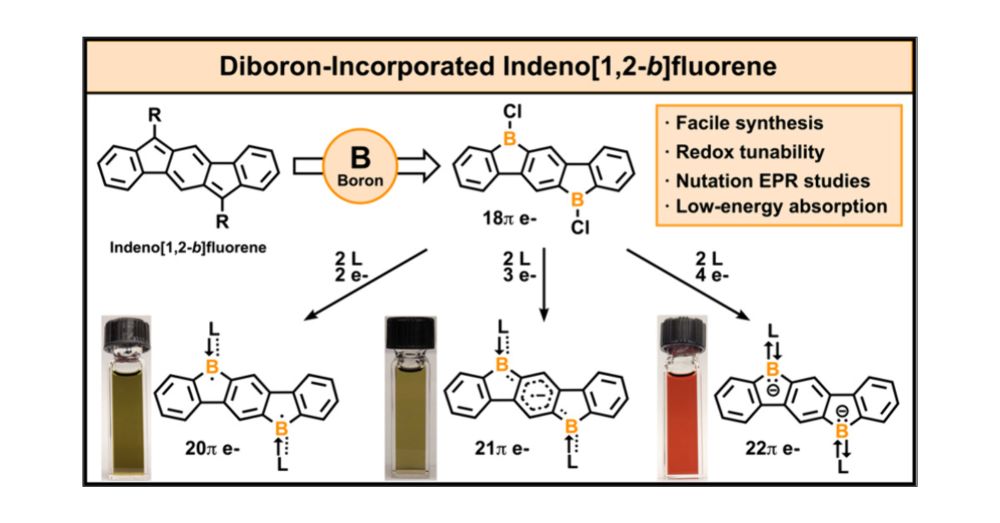
Diboron-Incorporated Indenofluorene: Isolation of Crystalline Neutral and Reduced States of 6,12-Diboraindeno[1,2-b]fluorene
The synthesis and redox transformations of 6,12-diboraindeno[1,2-b]fluorene (DBIF)─a pentacyclic π-system with diboron incorporation─are reported. In notable contrast to the all-hydrocarbon indenofluo...
pubs.acs.org
Gilliard Group
@gilliardgroup.bsky.social
· Mar 25
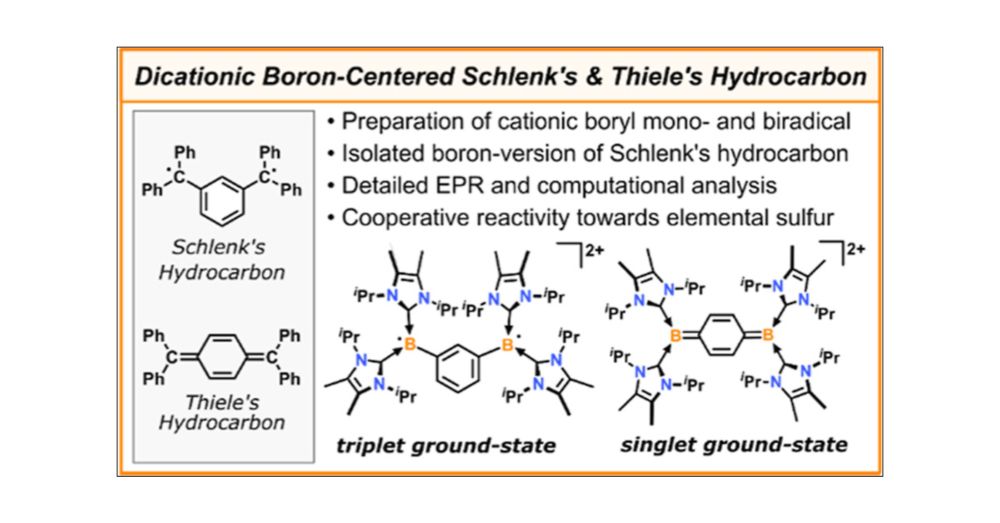
Dicationic Boron Derivatives of Schlenk’s and Thiele’s Hydrocarbon
In recent years, neutral NHC-stabilized boryl radicals have been investigated as reactive species in various organic transformations. However, cationic boron radicals have been significantly less explored. In addition, boron-centered open-shell species with S > 1/2 have emerged as attractive synthetic targets. In this study, we provide a synthetic route to an NHC-stabilized boryl radical cation as a salt of the weakly coordinating [Al(ORF)4]− (RF = C(CF3)3) anion. The synthetic procedure was extended to dicationic diboron derivatives of Schlenk’s and Thiele’s hydrocarbons with meta- and para-phenylene coupling units between the spin centers. While most known isolable boron biradicals have a singlet ground-state with a thermally accessible triplet state, the boron version of Schlenk’s hydrocarbon occupies a ground-state triplet spin-state, as shown by combined electron paramagnetic resonance spectroscopy and density functional theory studies. Furthermore, initial reactivity studies of the dications with elemental sulfur and diphenyldiselenide are presented.
pubs.acs.org
Gilliard Group
@gilliardgroup.bsky.social
· Dec 25