Hannah R. Abrams
@hannahrabrams.bsky.social
2.2K followers
350 following
85 posts
Heme/Onc fellow hoping to reduce costs & improve patient/caregiver experiences in cancer care. Views are my own. 🏳️🌈 #MedSky
Posts
Media
Videos
Starter Packs
Reposted by Hannah R. Abrams
Reposted by Hannah R. Abrams
Reposted by Hannah R. Abrams
Reposted by Hannah R. Abrams
Reposted by Hannah R. Abrams
Reposted by Hannah R. Abrams
Reposted by Hannah R. Abrams
Reposted by Hannah R. Abrams
Reposted by Hannah R. Abrams
Stacie Dusetzina
@dusetzinas.bsky.social
· May 16
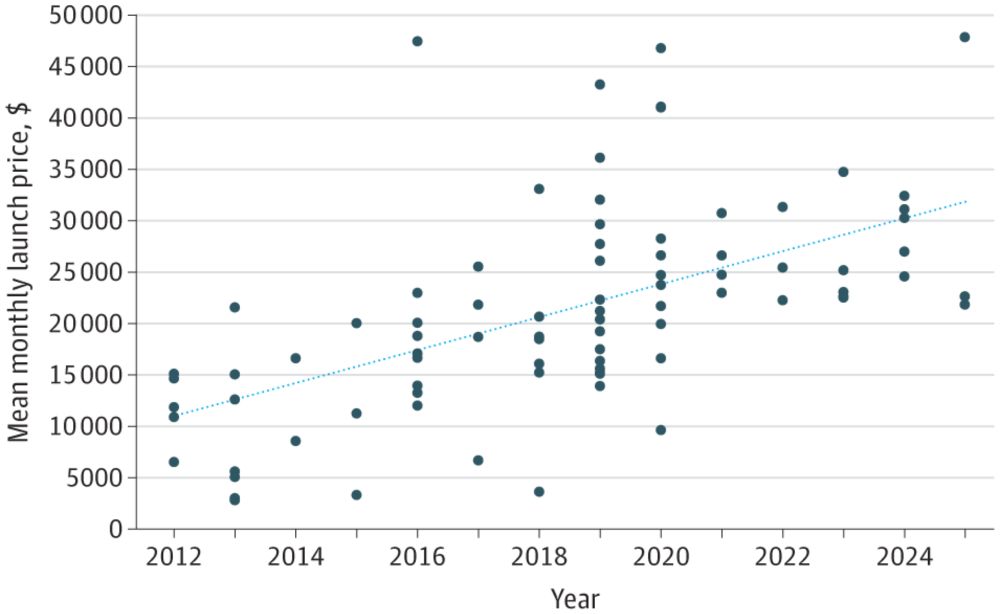
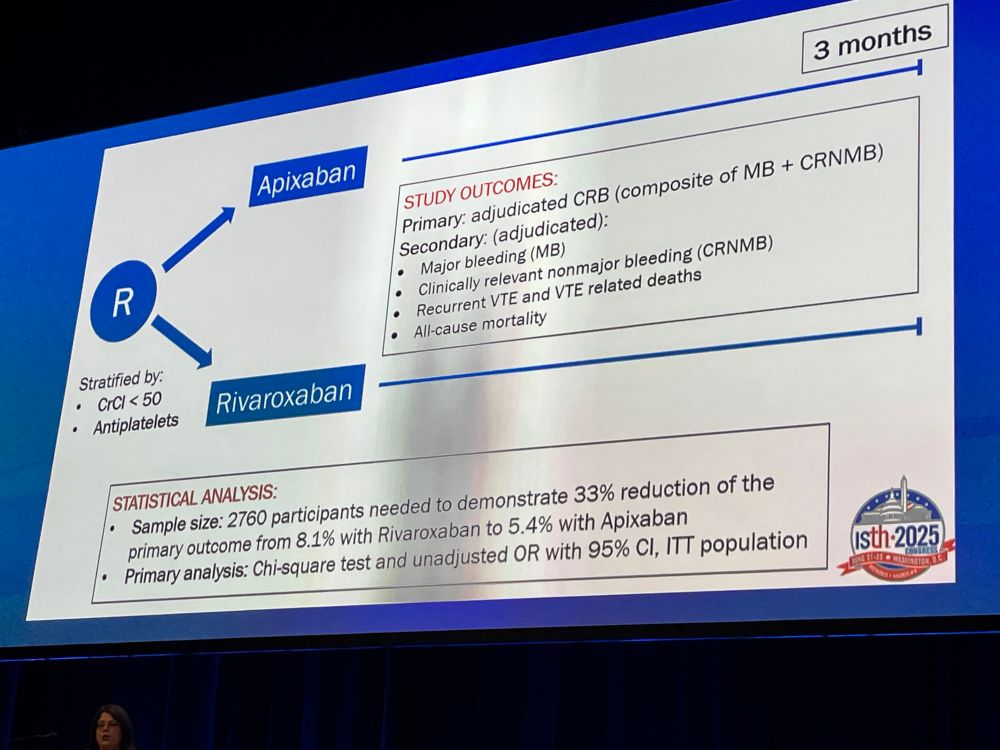
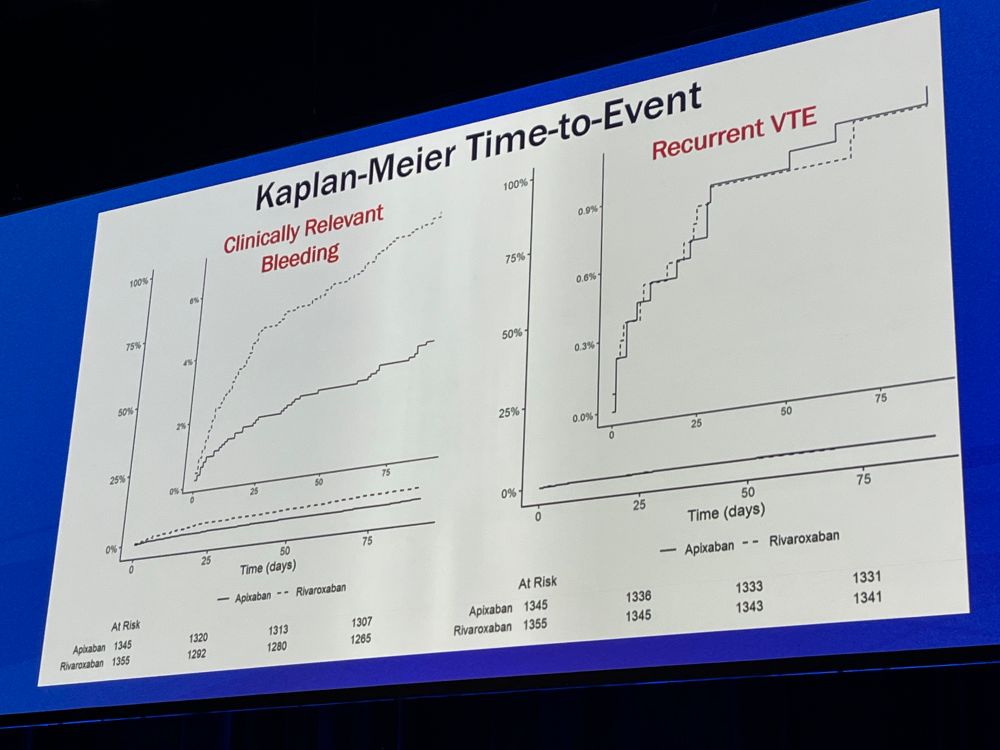
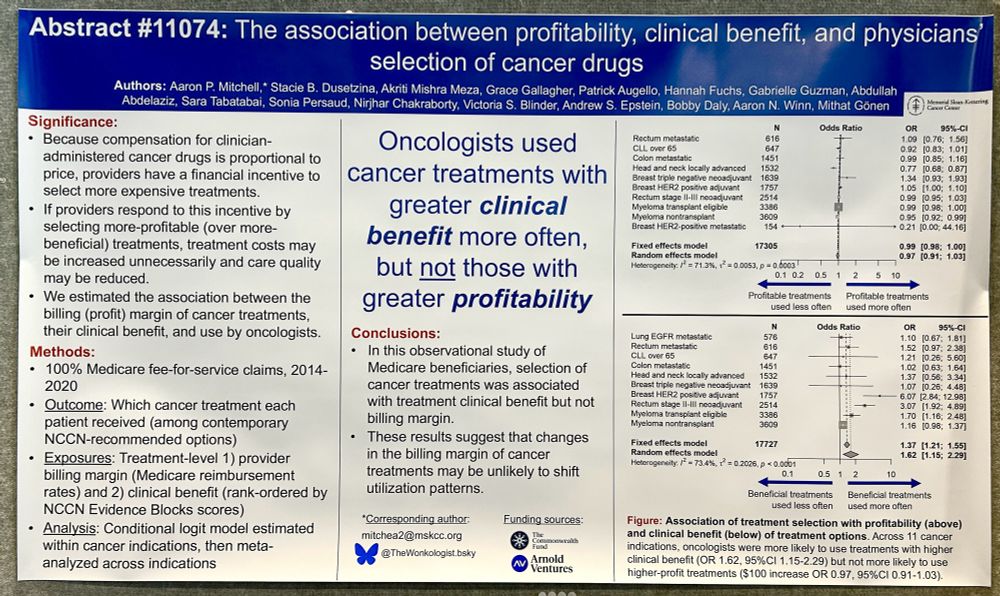
Trends in Launch Prices and Price Increases for Self-Administered Anticancer Drugs in Medicare
This study examines trends in launch prices and price increases for anticancer therapies covered under Medicare Part D and approved from 2010 to 2024.
jamanetwork.com
Reposted by Hannah R. Abrams
Reposted by Hannah R. Abrams
Reposted by Hannah R. Abrams