Imo Squire
@imosquire.bsky.social
93 followers
150 following
5 posts
PhD student in Bakewell group at KCL | Main group chemistry | Uni of York alumna | she/her
Posts
Media
Videos
Starter Packs
Reposted by Imo Squire
Reposted by Imo Squire
Reposted by Imo Squire
Reposted by Imo Squire
Reposted by Imo Squire
Reposted by Imo Squire
Robert Mulvey
@remglasgow.bsky.social
· Aug 28
Reposted by Imo Squire
Fabian Dankert
@fabiandankert.bsky.social
· Aug 25

Functional Al/Cd Heterometallics─From Controlled Al(I) Transfer to Nucleophilic Transfer of Cadmium Ions
Low-valent cadmium compounds have remained largely unexplored as electron reservoirs, with no precedent for their use in reduction or bond activation chemistry. Here, we address this gap by integratin...
pubs.acs.org
Reposted by Imo Squire
Robin Fulton
@fultonro.bsky.social
· Aug 12

Reductive Deamination of a Diaminogermylene Promoted by an Aluminyl Anion: Isolation of Ge(I), Ge(0) and Ge(‐I) Products
The potassium aluminyl K[Al(NON)] reacts with Ge[N(SiMe3)2]2 by a sequential reductive deamination pathway. The initially formed aluminacyclodigermene contains Ge(I) centres and may be considered as ...
onlinelibrary.wiley.com
Reposted by Imo Squire
Kyle Pearce
@kylegpearce.bsky.social
· Aug 2
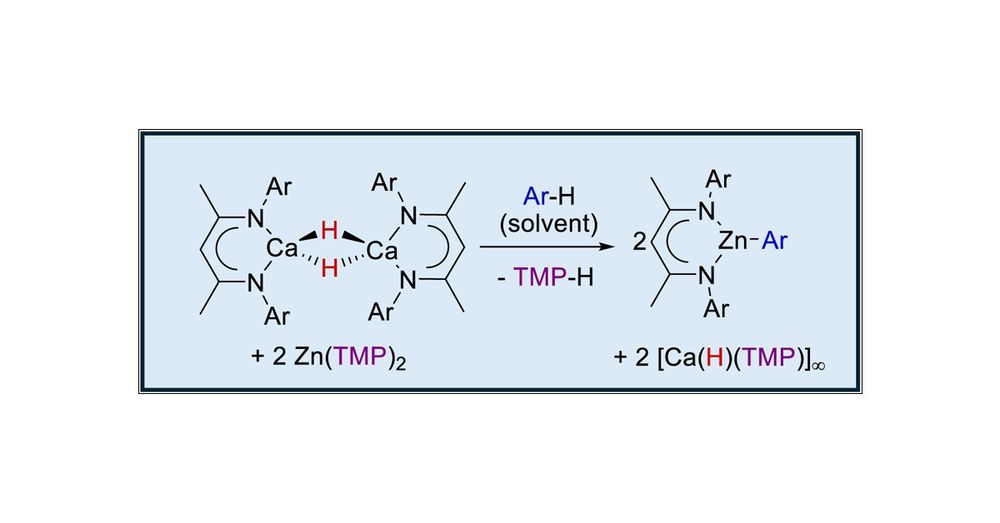
Arene Activation by Calcium Hydride/Zinc Amide Equilibration
The β-diketiminato calcium hydride, [(BDI)CaH]2 (BDI = HC{(Me)CNDipp}2, where Dipp = 2,6-i-Pr2C6H3), reacts with [Zn{N(SiMe3)2}2] and [Zn(TMP)2] (TMP = 2,2,6,6-tetramethylpiperidide) to provide labile...
pubs.acs.org
Reposted by Imo Squire
Reposted by Imo Squire
Reposted by Imo Squire
Reposted by Imo Squire
Kyle Pearce
@kylegpearce.bsky.social
· May 9
Mechanochemically Micronised Na/NaCl; a Superfine Reductant
A free-flowing, homogeneous and non-pyrophoric powder of Na/NaCl is prepared via planetary ball-milling. The mechanochemically micronised Na/NaCl serves as a highly activated source of sodium and was ...
pubs.rsc.org
Reposted by Imo Squire
Neal Mankad
@metallacycle.bsky.social
· May 8

Molecular Design of Al(II) Intermediates for Small Molecule Activation
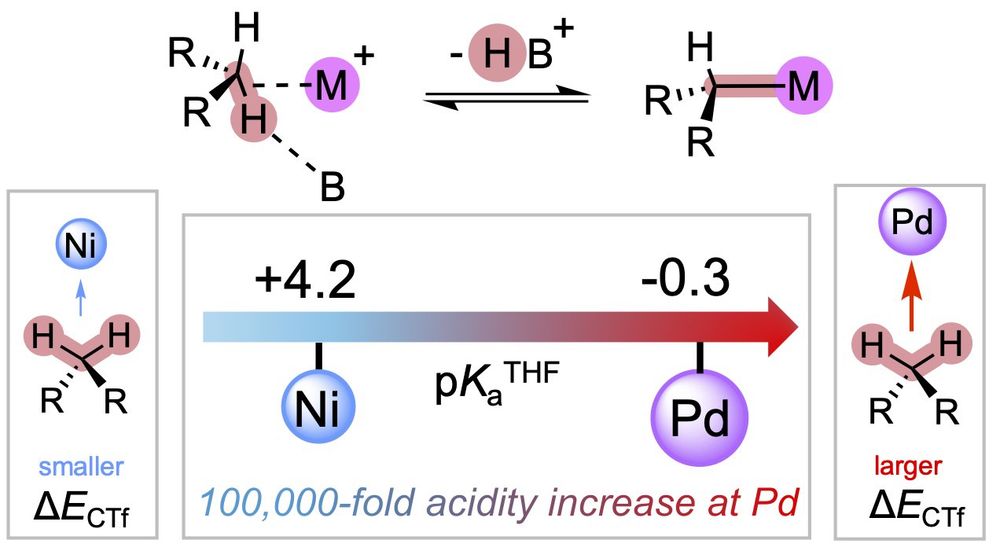
Promoting societally important small molecule activation processes with earth-abundant metals is foundational for a sustainable chemistry future. In this context, mapping new reaction pathways that would enable abundant main-group elements to mimic the behaviors of d- and f-block elements is facilitated by exploring unusual oxidation states. The most abundant metal on earth, aluminum, has been well studied in the Lewis acidic +III and Lewis basic +I oxidation states but rarely in the potentially biphilic +II oxidation state until recently, when a renaissance of Al(II) chemistry emerged from a range of research groups. In this Perspective, we review the chemistry of mononuclear Al radicals, including both Al-centered radicals (i.e., Al(II) compounds) and redox non-innocent systems (i.e., formally Al(II) species that are physically Al(III) with ligand-centered radicals), with an emphasis on small molecule reactivity. We also provide a meta-analysis of the Al(II) literature to summarize how different design strategies (e.g., redox non-innocence, strained coordination geometries) have been shown to impart biphilic character to Al radicals and tune their behavior, thus allowing Al radicals to mimic the chemistry of certain d- and f-block metal ions such as Ti(III) and Sm(II). We expect these molecular design concepts to inform future Al(II) studies as the chemistry of this unusual oxidation state of Al continues to grow.
doi.org
Reposted by Imo Squire
Reposted by Imo Squire
Reposted by Imo Squire
Matt de Vere
@mattdv-t.bsky.social
· Apr 16
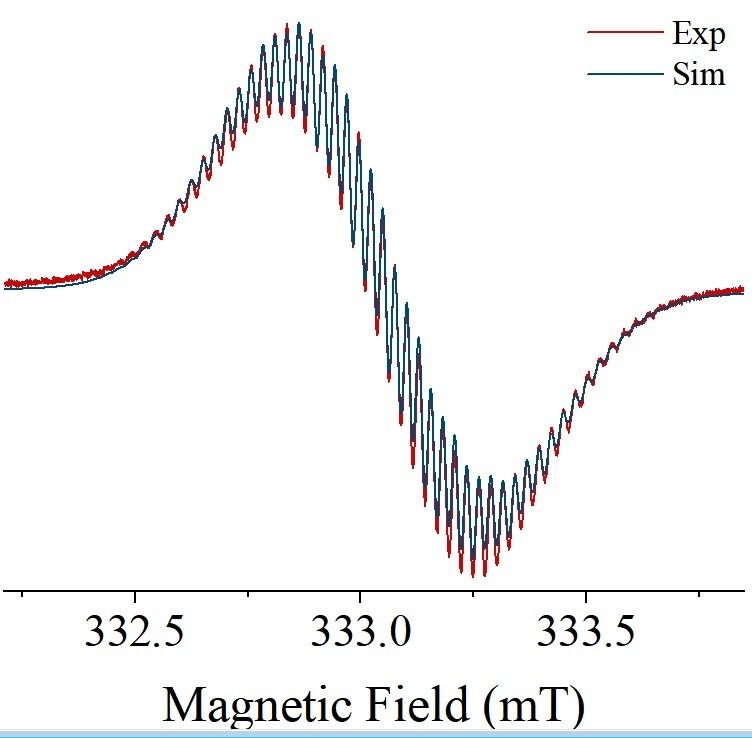
Reversible addition of ethene to gallium(I) monomers and dimers
Reversible interactions of organic substrates with transition metal compounds are a hallmark of their chemistry and its catalytic applications, but remain uncommon for low-valent p-block compounds. We...
pubs.rsc.org