James Bull
@jamesabull.bsky.social
430 followers
230 following
17 posts
Professor of Synthetic Chemistry at Imperial College London
Posts
Media
Videos
Starter Packs
James Bull
@jamesabull.bsky.social
· Aug 13

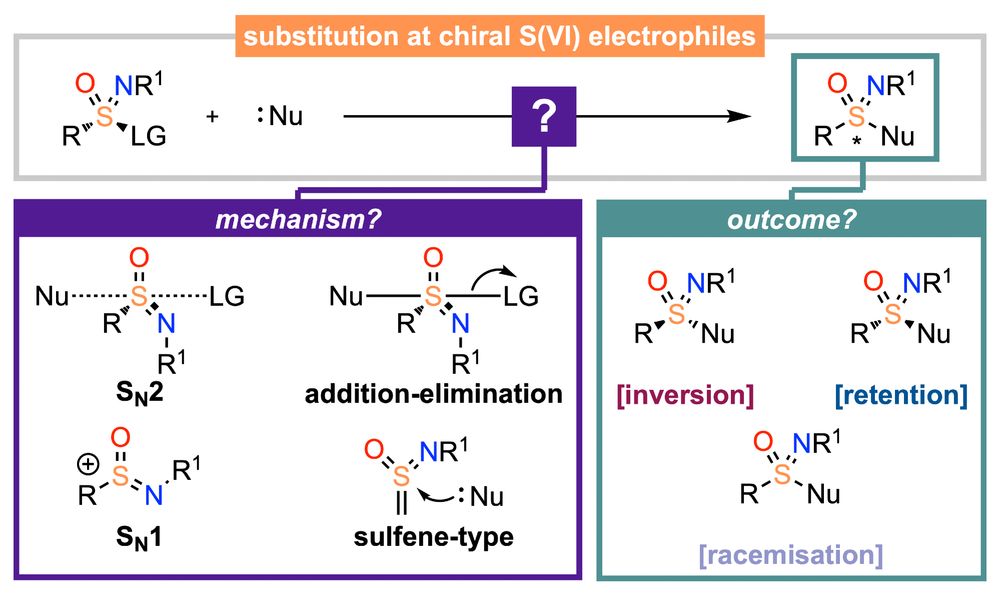
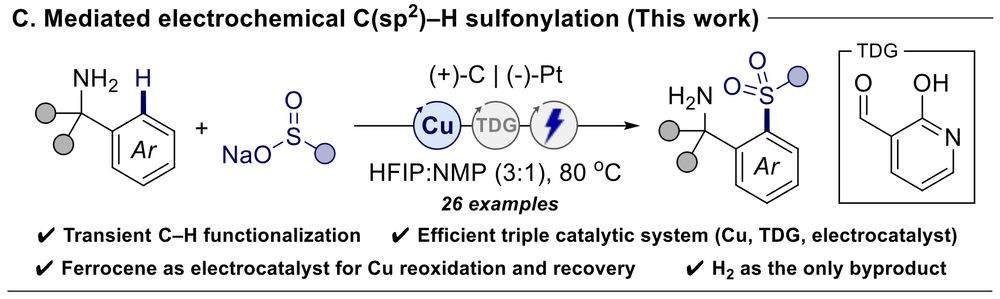
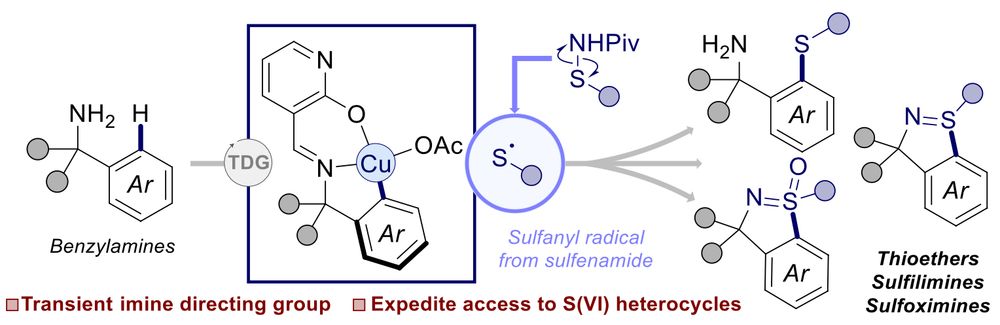
Synthesis of Cyclic Sulfilimines and Sulfoximines via Copper‐Mediated C(sp2)–H Sulfanylation of Benzylamines with a Catalytic Transient Directing Group
Copper-mediated transient C─H sulfanylation of benzylamines with sulfenamides enables rapid access to thioethers, cyclic sulfilimines, and sulfoximines. Mechanistic investigations using 1H NMR and el....
doi.org
James Bull
@jamesabull.bsky.social
· Jul 11
James Bull
@jamesabull.bsky.social
· Jul 11
James Bull
@jamesabull.bsky.social
· May 19

Catalytic SuFEx Reactivity of Sulfonimidoyl Fluorides with Functionalized Amines with Automation Applicable Conditions
Sulfonimidamides have emerged as attractive chemical motifs in drug discovery and as sulfonamide bioisosteres that can offer improved pharmacokinetic and physiochemical properties. Here we report a mi...
doi.org
James Bull
@jamesabull.bsky.social
· May 13

Development of automated workflows for direct-to-biology drug discovery of kinase inhibitors at Imperial College London on FindAPhD.com
PhD Project - Development of automated workflows for direct-to-biology drug discovery of kinase inhibitors at Imperial College London, listed on FindAPhD.com
www.findaphd.com
Reposted by James Bull
Jess Pancholi
@jesspanch.bsky.social
· Feb 19
Reposted by James Bull
James Bull
@jamesabull.bsky.social
· Dec 16
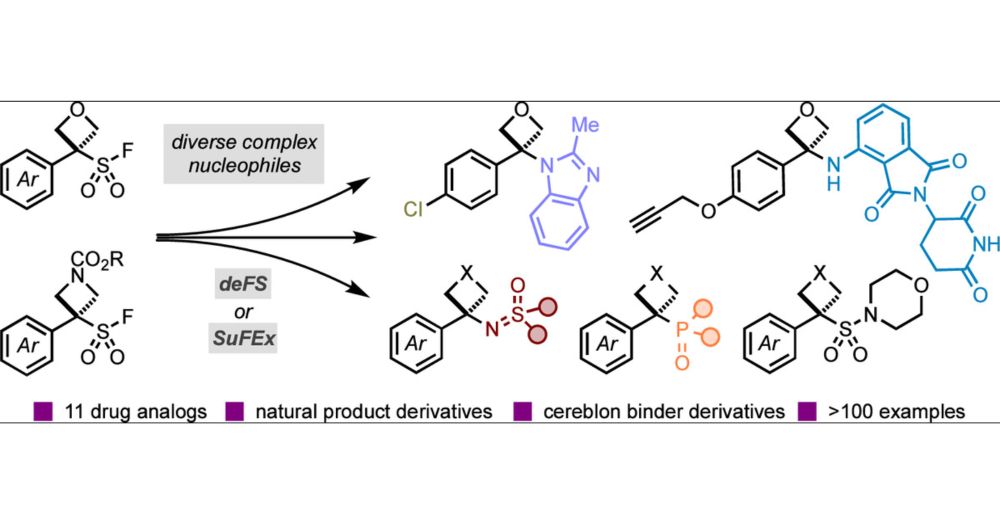
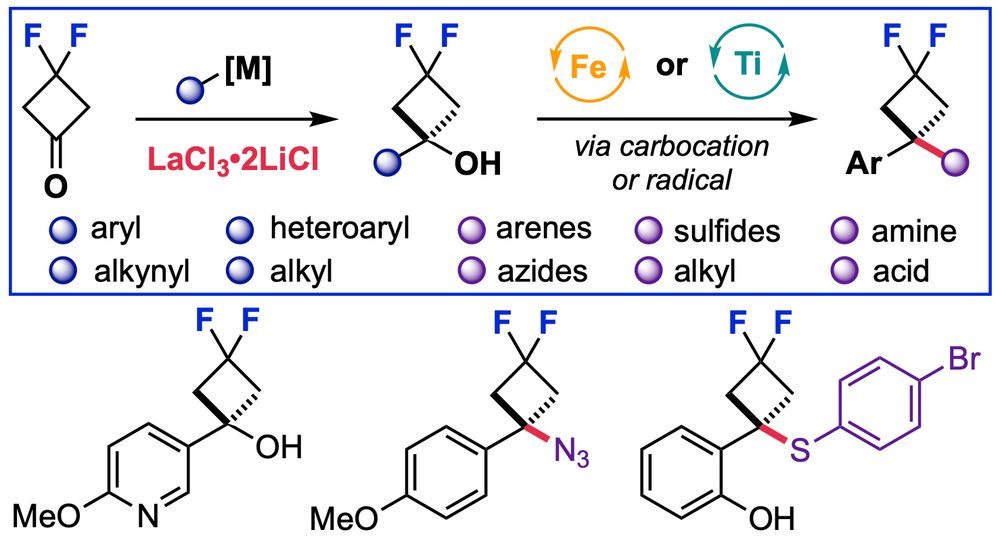
Harnessing Oxetane and Azetidine Sulfonyl Fluorides for Opportunities in Drug Discovery
Four-membered heterocycles such as oxetanes and azetidines represent attractive and emergent design options in medicinal chemistry due to their small and polar nature and potential to significantly impact the physiochemical properties of drug molecules. The challenging preparation of these derivatives, especially in a divergent manner, has severely limited their combination with other medicinally and biologically important groups. Consequently, there is a substantial demand for mild and effective synthetic strategies to access new oxetane and azetidine derivatives and molecular scaffolds. Here, we report the development and use of oxetane sulfonyl fluorides (OSFs) and azetidine sulfonyl fluorides (ASFs), which behave as precursors to carbocations in an unusual defluorosulfonylation reaction pathway (deFS). The small-ring sulfonyl fluorides are activated under mild thermal conditions (60 °C), and the generated reactive intermediates couple with a broad range of nucleophiles. Oxetane and azetidine heterocyclic, -sulfoximine, and -phosphonate derivatives are prepared, several of which do not have comparable carbonyl analogs, providing new chemical motifs and design elements for drug discovery. Alternatively, a SuFEx pathway under anionic conditions accesses oxetane-sulfur(VI) derivatives. We demonstrate the synthetic utility of novel OSF and ASF reagents through the synthesis of 11 drug analogs, showcasing their potential for subsequent diversification and facile inclusion into medicinal chemistry programs. Moreover, we propose the application of the OSF and ASF reagents as linker motifs and demonstrate the incorporation of pendant groups suitable for common conjugation reactions. Productive deFS reactions with E3 ligase recruiters such as pomalidomide and related derivatives provide new degrader motifs and potential PROTAC linkers.
doi.org