James Birrell
@jamesbirrell.bsky.social
49 followers
33 following
24 posts
Lecturer at University of Essex. Interested in metalloenzyme structure and spectroscopy.
Posts
Media
Videos
Starter Packs
Reposted by James Birrell
ICH2026
@ich2026.bsky.social
· Jul 23
James Birrell
@jamesbirrell.bsky.social
· Jun 27
James Birrell
@jamesbirrell.bsky.social
· Jun 27
James Birrell
@jamesbirrell.bsky.social
· Jun 27
Reposted by James Birrell
Reposted by James Birrell
Sven T. Stripp
@stripplab.bsky.social
· May 22
Reposted by James Birrell
Sven T. Stripp
@stripplab.bsky.social
· May 22
James Birrell
@jamesbirrell.bsky.social
· May 22
Reposted by James Birrell
Reposted by James Birrell
Reposted by James Birrell
Reposted by James Birrell
Reposted by James Birrell
Reposted by James Birrell
Reposted by James Birrell
Sandy Kilpatrick
@sandykilla.bsky.social
· May 21
Reposted by James Birrell
Reposted by James Birrell
Reposted by James Birrell
James Birrell
@jamesbirrell.bsky.social
· May 14
James Birrell
@jamesbirrell.bsky.social
· May 14
Reposted by James Birrell
Rodriguez-Macia Lab
@rmlab.bsky.social
· May 11
James Birrell
@jamesbirrell.bsky.social
· May 10
Reposted by James Birrell
Rodriguez-Macia Lab
@rmlab.bsky.social
· May 11
James Birrell
@jamesbirrell.bsky.social
· May 10
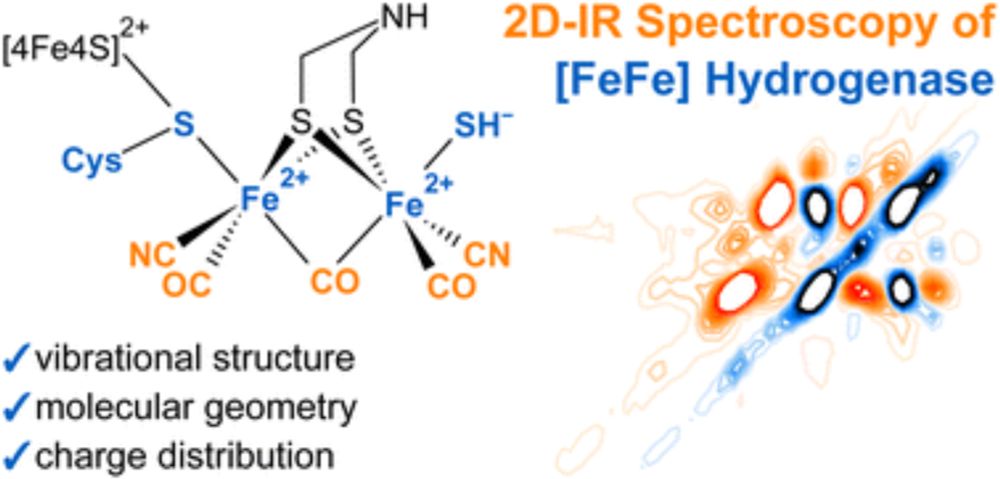
Two-dimensional Infrared Spectroscopy as a Tool to Reveal the Vibrational and Molecular Structure of [FeFe] Hydrogenases
[FeFe] hydrogenases are Nature’s most efficient catalysts for the cleavage and evolution of molecular hydrogen. Despite decades of research, key aspects of the catalytic cycle and the underlying geome...
pubs.rsc.org
James Birrell
@jamesbirrell.bsky.social
· May 10
James Birrell
@jamesbirrell.bsky.social
· May 10