Dr. Jeffrey Millman
@jeffreymillman.bsky.social
1.8K followers
3.5K following
120 posts
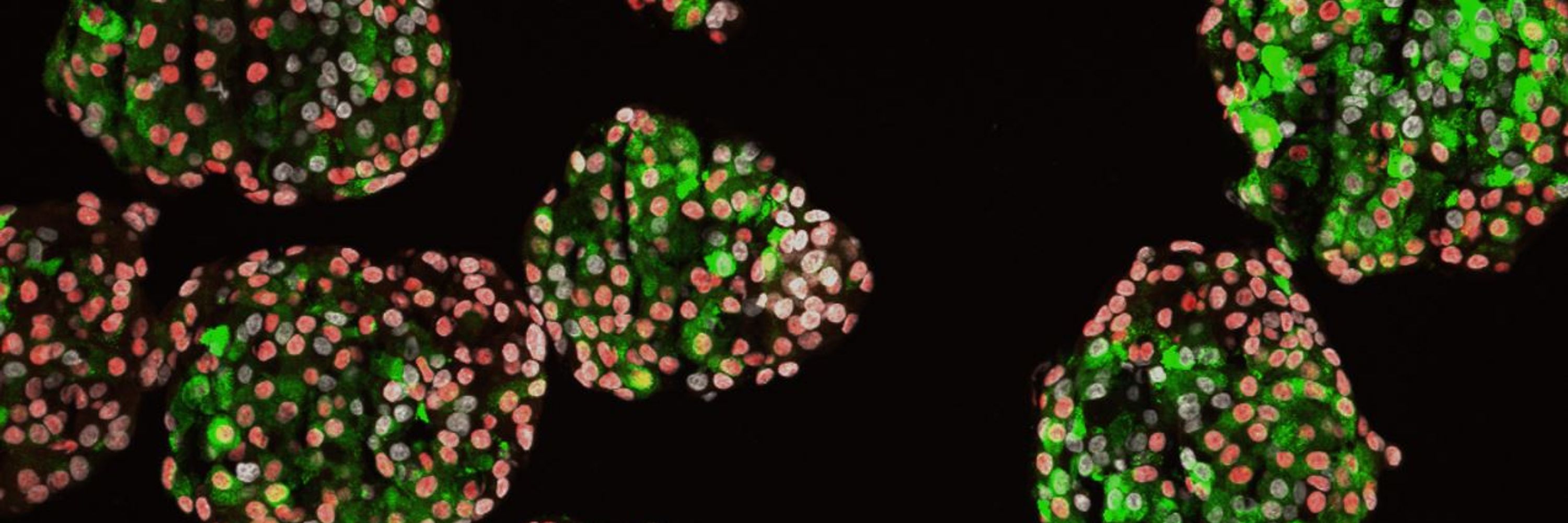
Washington University School of Medicine Professor | Diabetes Researcher | Stem Cell Biologist | Bioengineer | Inventor | STEM Educator | Former MIT & Harvard | Former Biotech VP | https://sites.wustl.edu/millmanlab/
Posts
Media
Videos
Starter Packs
Reposted by Dr. Jeffrey Millman
Nate Huebsch
@natehuebsch.bsky.social
· Jun 26

Enhancing the Potency of Growth Factor‐Mimicking Peptides via Cross‐Presentation With Integrin Ligands
Growth factors enhance survival and integration of transplanted Mesenchymal Stromal Cells (MSC), but successful supplementation often requires supraphysiological growth factor doses, risking off-targ....
dx.doi.org