Kazunari Miyamichi
@k-miyamichi.bsky.social
190 followers
250 following
50 posts
Team leader, RIKEN BDR, Kobe, Japan https://cco.riken.jp/index_en.html; Neuroscientist studying hypothalamus and sympathetic nervous systems in mice https://scholar.google.com/citations?user=yQZ8MFgAAAAJ; Any opinions expressed herein are personal.
Posts
Media
Videos
Starter Packs
Reposted by Kazunari Miyamichi
Neuron
@cp-neuron.bsky.social
· Feb 24

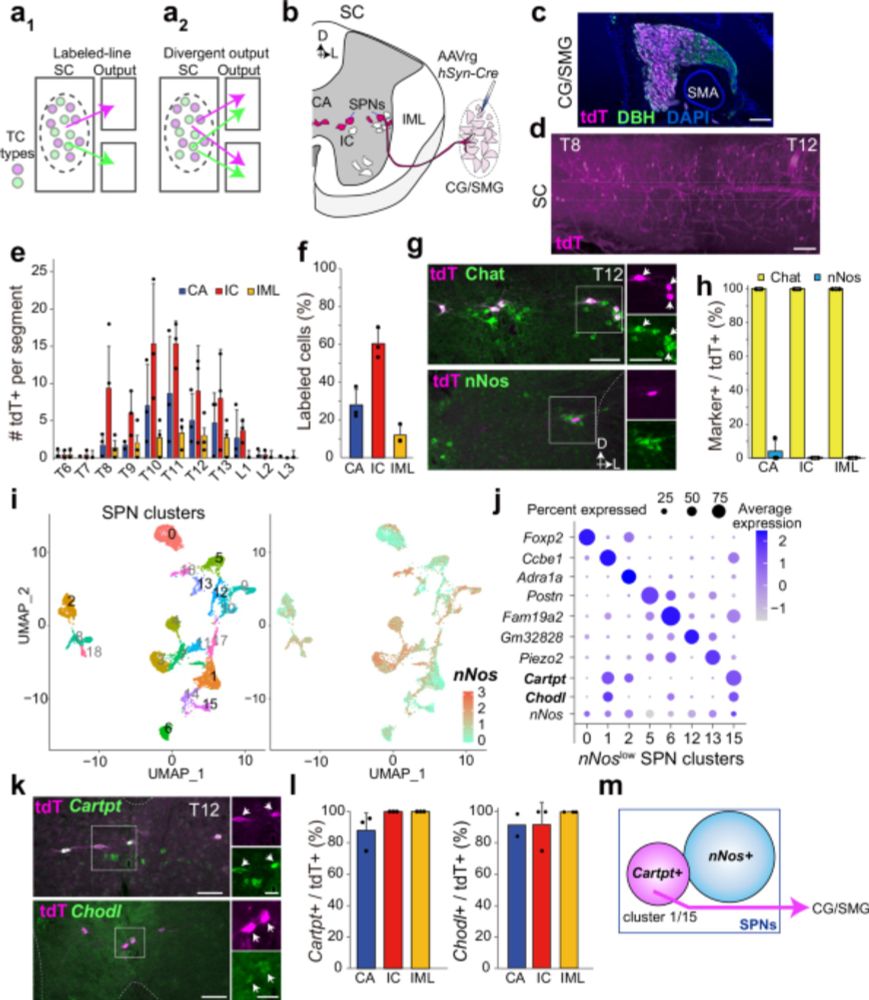
Dietary availability acutely influences puberty onset via a hypothalamic neural circuit
Goto et al. measure the pulsatile activity of the reproductive center in the hypothalamic arcuate nucleus during puberty in female mice. They discover that food availability rapidly and bidirectionally modulates this pulsatile activity, partially through…
dlvr.it
Reposted by Kazunari Miyamichi
Fumiaki Obata
@obataf-lab.bsky.social
· Dec 24

A nonsecretory antimicrobial peptide mediates inflammatory organ damage in Drosophila renal tubules
Using the power of Drosophila genetics, Oi et al. identified a nonsecretory antimicrobial peptide, Attacin-D, as an essential factor in inflammatory organ damage in the renal tubules. This study highlights a key player in inflammatory organ injury.
www.cell.com
Reposted by Kazunari Miyamichi
Kota Mizumoto
@kota-mizumoto.bsky.social
· Dec 23

A modular system to label endogenous presynaptic proteins using split fluorophores in C. elegans
Abstract. Visualizing the subcellular localization of presynaptic proteins with fluorescent proteins is a powerful tool to dissect the genetic and molecula
academic.oup.com