Lukas Goede
@lukasgoede.bsky.social
710 followers
400 following
44 posts
Neuroscientist @ Brigham & Women's Hospital, Harvard Medical School | @netstim.org | Neurology resident | Interested in deep brain stimulation, non-invasive brain stimulation and movement disorders
Posts
Media
Videos
Starter Packs
Reposted by Lukas Goede
Lukas Goede
@lukasgoede.bsky.social
· May 22
Lukas Goede
@lukasgoede.bsky.social
· May 22
Lukas Goede
@lukasgoede.bsky.social
· May 22
Lukas Goede
@lukasgoede.bsky.social
· May 22
Lukas Goede
@lukasgoede.bsky.social
· May 22
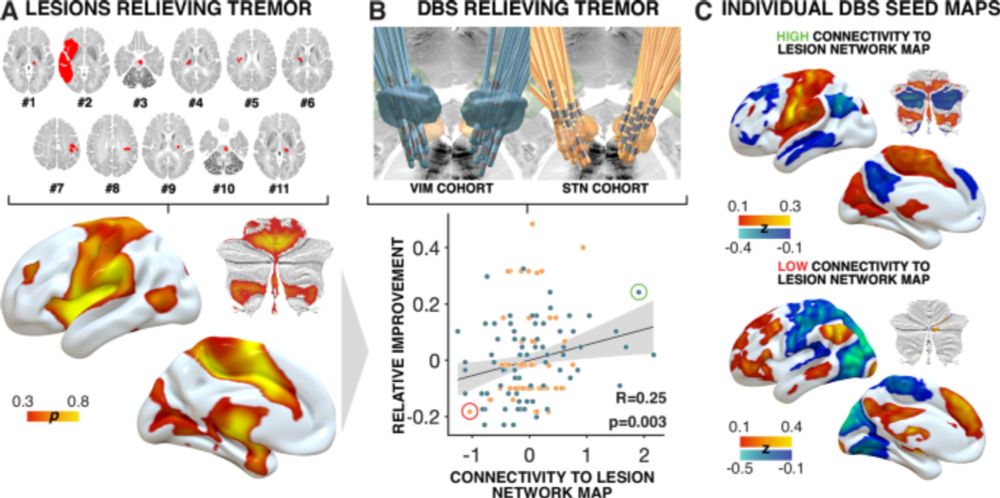
Convergent mapping of a tremor treatment network - Nature Communications
Goede et al. combined multiple modalities to define a common tremor network across disorders. This finding may help optimize deep brain stimulation and guide future noninvasive therapies.
www.nature.com
Lukas Goede
@lukasgoede.bsky.social
· May 22
Lukas Goede
@lukasgoede.bsky.social
· May 22
Lukas Goede
@lukasgoede.bsky.social
· May 22
Lukas Goede
@lukasgoede.bsky.social
· May 22
Lukas Goede
@lukasgoede.bsky.social
· May 22

Mapping Essential Tremor to a Common Brain Network Using Functional Connectivity Analysis | Neurology
Background and ObjectivesThe cerebello-thalamo-cortical circuit plays a critical role in essential tremor (ET).
However, abnormalities have been reported in multiple brain regions outside this circuit...
doi.org
Lukas Goede
@lukasgoede.bsky.social
· May 22

Identifying therapeutic targets from spontaneous beneficial brain lesions
Brain damage can occasionally result in paradoxical functional benefit, which could help identify therapeutic targets for neuromodulation. However, these beneficial lesions are rare and lesions in mu...
doi.org
Lukas Goede
@lukasgoede.bsky.social
· May 22
Lukas Goede
@lukasgoede.bsky.social
· May 19

Patient Selection in Deep Brain Stimulation: A Role for Transcranial Direct Current Stimulation to Enhance the Levodopa Challenge?
Dopaminergic medication and deep brain stimulation (DBS) improve motor symptoms in Parkinson's disease (PD), but levodopa response alone may not predict DBS outcomes. We retrospectively analyzed 19 P....
onlinelibrary.wiley.com
Lukas Goede
@lukasgoede.bsky.social
· May 19