Luo Lab
@luolab-utah.bsky.social
290 followers
240 following
30 posts
The Interdisciplinary Electrochemistry Group at the University of Utah
Website: https://luo.chem.utah.edu/
Posts
Media
Videos
Starter Packs
Reposted by Luo Lab
Luo Lab
@luolab-utah.bsky.social
· Aug 22
Utah Chemistry
@utahchemistry.bsky.social
· Aug 21

Chemistry Department Hosts First Utah Electrochemistry Symposium - Department of Chemistry
Profs. Henry White, Shelley Minteer, and Long Luo hosted the first Utah Electrochemistry Symposium (UTES) on July 25–26, 2025, at the University of Utah’s Department...
www.chemistry.utah.edu
Luo Lab
@luolab-utah.bsky.social
· Aug 5
Luo Lab
@luolab-utah.bsky.social
· Jul 21
Luo Lab
@luolab-utah.bsky.social
· Jul 21
Origin of Selectivity in Alternating Current-Enabled Partial Reduction of (Hetero)Arenes: A Case Study of Two Consecutive Irreversible Electrochemical Steps
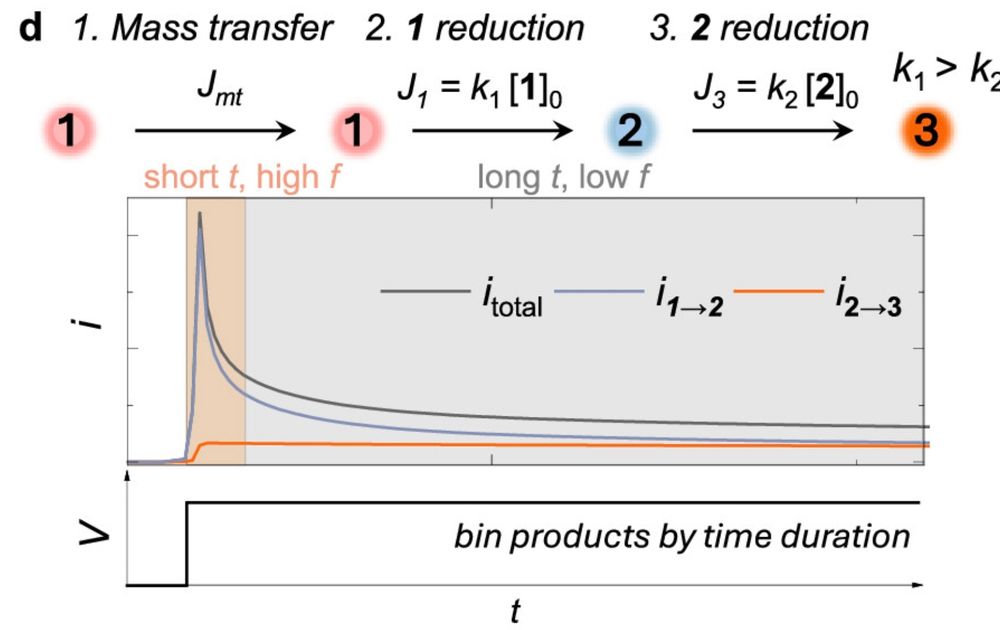
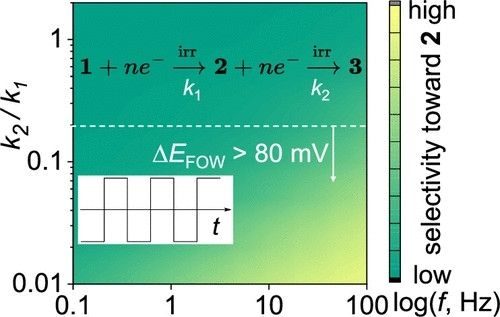
Herein, we investigate the origin of selectivity in the alternating current (AC)-enabled partial reduction of (hetero)arenes to cyclic alkenes. Reduction of (hetero)arenes can be considered as a reaction involving two consecutive irreversible electrochemical steps: the first generates the desired cyclic alkene, while the second leads to its undesired overreduction. Conventional constant current or voltage (DC) electrolysis results in poor selectivity toward the partial reduction products, originating from overreduction and base-induced decomposition of the desired product. Fast-scan cyclic voltammetry shows that the rate constant for the first reduction (k1) exceeds that of the second one (k2). Finite element simulations based on this experimental finding semiquantitatively capture the frequency-dependent selectivity observed in AC electrolysis experiments (i.e., increasing the AC frequency enhances selectivity). The results further reveal that AC electrolysis mitigates the low selectivity by only collecting the products at the initial stage of the reduction reaction, which is mostly under a kinetically controlled regime. We then extend the finite element model and introduce ΔEFOW, the foot-of-the-wave potential difference between cyclic voltammograms of substrate and partial reduction product, as an accessible proxy for k2/k1. A ΔEFOW > 80 mV predicts synthetically useful selectivity (>30%) toward the partial reduction product below 100 Hz.
pubs.acs.org
Luo Lab
@luolab-utah.bsky.social
· Jun 11
Luo Lab
@luolab-utah.bsky.social
· Jan 22
Challenges in PFAS Postdegradation Analysis: Insights from the PFAS-CTAB Model System
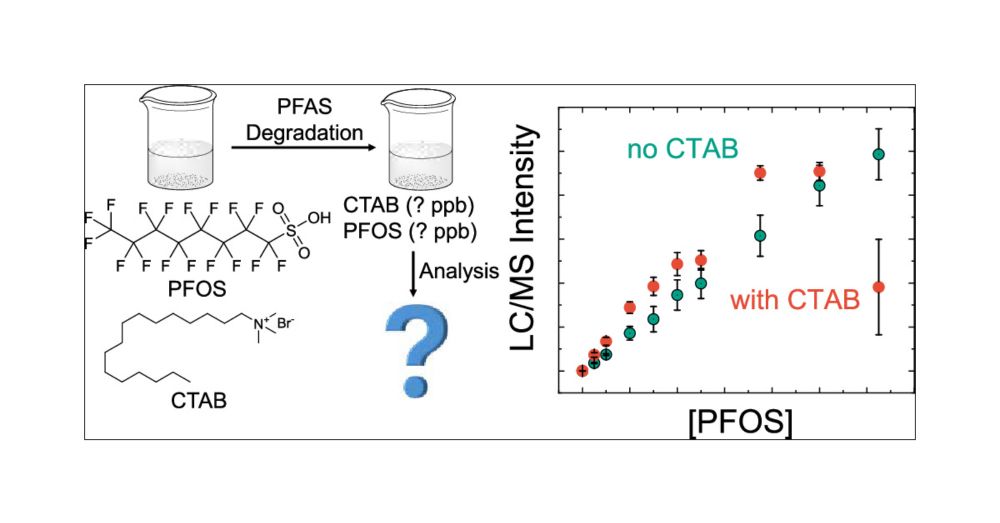
Per- and polyfluoroalkyl substances (PFAS) are synthetic chemicals widely used for their oil and water-repellent properties. Their environmental persistence and potential health risks have raised significant concerns. As PFAS degrades through remediation or natural processes, they form complex mixtures of the original chemicals, transformation byproducts, and degradation additives. Analyzing PFAS after degradation presents analytical challenges due to possible chemical and physical interactions, including ion pairing, micelle formation, and complexation. These factors can significantly impact the precision and accuracy of PFAS measurements, yet they are often overlooked in PFAS degradation studies. In this work, we demonstrate that with the addition of ppb-level cetyltrimethylammonium bromide (CTAB), a cationic surfactant used in PFAS plasma-based degradation, the PFAS calibration curve linearity, sensitivity, and reproducibility are severely compromised. Isotopically labeled internal standards cannot fully correct these issues. Furthermore, the standard EPA methods 537.1, 533, and 1633 could not accurately recover PFAS concentrations in the PFAS and CTAB mixtures, with severe matrix effects observed for longer-chain and nitrogen-containing PFAS. Among these methods, Method 1633 is currently the most suitable option for postdegradation analysis. Method 1633 showed the lowest CTAB interference because this method used another weak ion pair additive, formic acid or acetic acid (in commercial lab analysis), to acidify the sample before LC–MS/MS analysis and added an isotopically labeled internal standard. For future PFAS degradation studies, we recommend systematically evaluating the matrix effect on the PFAS quantification using a recovery matrix to validate the analytical methods before use.
pubs.acs.org