Meagan Oakley
@meaganoakley.bsky.social
1.5K followers
320 following
120 posts
never met an actinide I didn't like
🇨🇦👩💻⚛ DKO Research Fellow and computational chemist @ University of Manchester
Posts
Media
Videos
Starter Packs
Pinned
Meagan Oakley
@meaganoakley.bsky.social
· Dec 19

Trends in Methanol-Solvated Actinide Ions and Actinide Expanded Porphyrin Complexes
Trivalent actinide expanded porphyrin complexes have been of synthetic interest since the isolation of the series of trivalent lanthanide texaphyrin complexes in 1992, however, synthesis of these actinide-based complexes has not yet been achieved. In this work, a computational study with relativistic density functional theory was performed to determine how trivalent actinide ions (Ac3+ through Lr3+) interact with Schiff base expanded porphyrin macrocycles in a methanol solvent as an alternate pathway to stabilization. A thorough analysis of structural parameters, electronic structure, stability of microsolvation environments, and relative binding energies provided insight into the most stable structures. Trends in bonding and structure for the full actinide series are reported for methanol solvated ions, which reports actinide contraction along the series for early and mid actinides. Texaphyrin incorporates the actinide ions in a planar fashion, whereas for alaskphyrin a bent structure is more favorable; this bend results in stronger interaction energies than those calculated for the texaphyrin complexes. We also show that the redox-active expanded porphyrin ligands are able to accommodate a variety of actinides through charge transfer stabilization. Based on relative binding energy and energy decomposition analysis, it was found that texaphyrin and alaskaphyrin both exhibit a strong preference for binding to Th.
pubs.acs.org
Reposted by Meagan Oakley
Reposted by Meagan Oakley
Reposted by Meagan Oakley
Meagan Oakley
@meaganoakley.bsky.social
· Aug 19
Meagan Oakley
@meaganoakley.bsky.social
· Aug 18
Reposted by Meagan Oakley
Reposted by Meagan Oakley
Richard Stupart
@richardstupart.com
· Jul 31
Reposted by Meagan Oakley
Reposted by Meagan Oakley
julie muncy
@julie.radiantarray.io
· Jul 14
Meagan Oakley
@meaganoakley.bsky.social
· Jul 13
Reposted by Meagan Oakley
Reposted by Meagan Oakley
David Mills
@millsgroupchem.bsky.social
· Jun 25
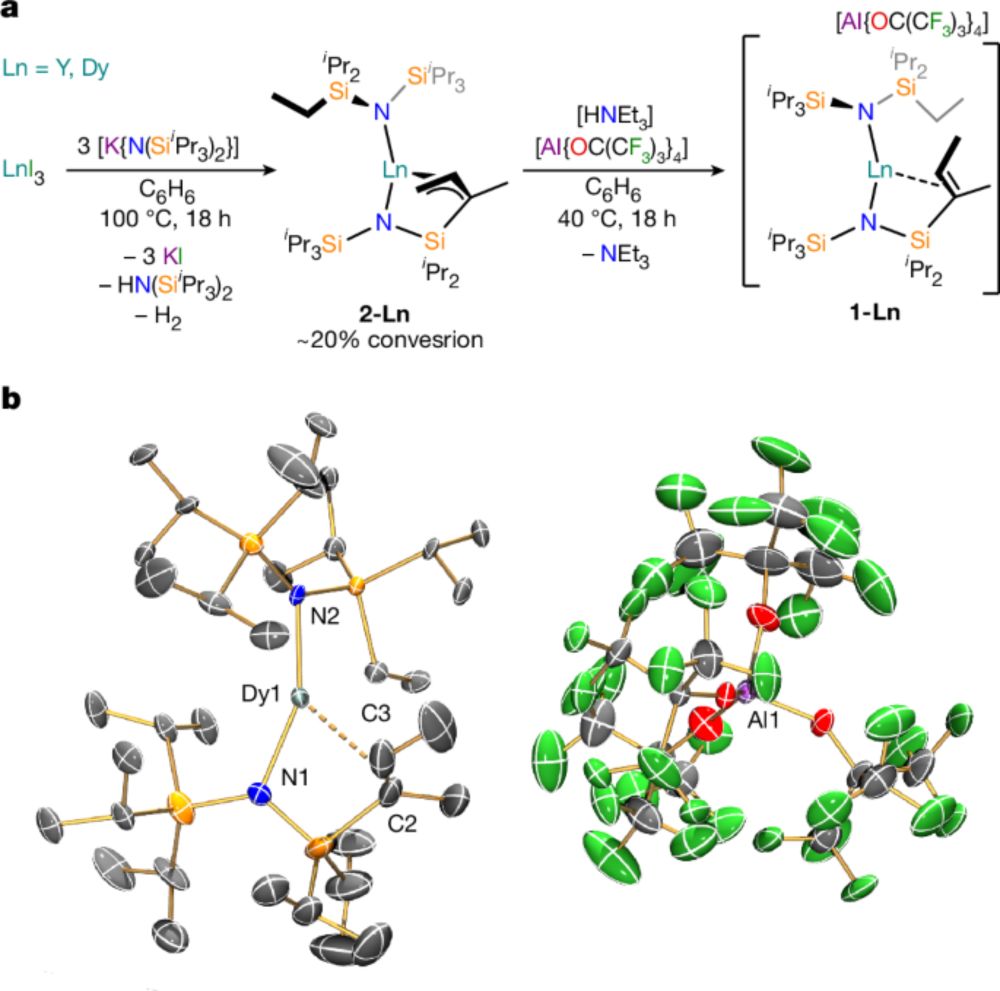
Soft magnetic hysteresis in a dysprosium amide–alkene complex up to 100 kelvin - Nature
A dysprosium amide–alkene complex shows soft magnetic hysteresis loops up to 100 kelvin, arising from the high charge density of the amide ligands and the structural role of the pendant alkene.
www.nature.com
Meagan Oakley
@meaganoakley.bsky.social
· Jun 17
Meagan Oakley
@meaganoakley.bsky.social
· Jun 17
Meagan Oakley
@meaganoakley.bsky.social
· Jun 17
Meagan Oakley
@meaganoakley.bsky.social
· Jun 17
Reposted by Meagan Oakley
Meagan Oakley
@meaganoakley.bsky.social
· Jun 13