Enrique Soto
@mrenrisoto.bsky.social
67 followers
100 following
6 posts
Postdoctoral researcher @goicoecheagroup.bsky.social. Working on some cool main-group chemistry!! #TheParamagneticGuy
Ph.D. (2025) from @camposgroup.bsky.social
B. Sc. and M. Sc. in Chemistry from @humboldtuni.bsky.social
Posts
Media
Videos
Starter Packs
Pinned
Enrique Soto
@mrenrisoto.bsky.social
· Dec 20
Reposted by Enrique Soto
Reposted by Enrique Soto
EL PAÍS
@elpais.com
· 5d

Las dos Paulas, Blasi y Ostiz, brindan a España un doble oro en el Europeo de ciclismo
La catalana, bronce mundial en Ruanda, conquista su primer título continental en categoría sub-23; la navarra, vigente maillot arcoíris júnior, encadena su segundo triunfo continental tras la contrarreloj del miércoles
elpais.com
Reposted by Enrique Soto
Reposted by Enrique Soto
Reposted by Enrique Soto
Enrique Soto
@mrenrisoto.bsky.social
· Aug 21
Reposted by Enrique Soto
Reposted by Enrique Soto
Debanik Ray
@debanikray.bsky.social
· Aug 18
Reposted by Enrique Soto
ChemComm
@chemcomm.rsc.org
· Aug 7
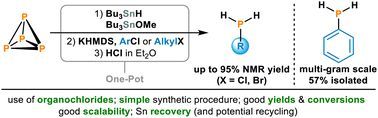
One-pot synthesis of primary phosphines from white phosphorus
Aryl and alkyl chlorides are inexpensive and readily accessible, making them ideal reagents for converting white phosphorus (P4) into primary phosphines RPH2. However, methods for achieving this…
buff.ly
Reposted by Enrique Soto
Pedro Vallín
@pvallin.bsky.social
· Aug 6
Enrique Soto
@mrenrisoto.bsky.social
· Aug 3
Reposted by Enrique Soto
Reposted by Enrique Soto
Reposted by Enrique Soto
Reposted by Enrique Soto
Reposted by Enrique Soto
Reposted by Enrique Soto
Ksenia Glusac
@ksenia-glusac.bsky.social
· Jun 24
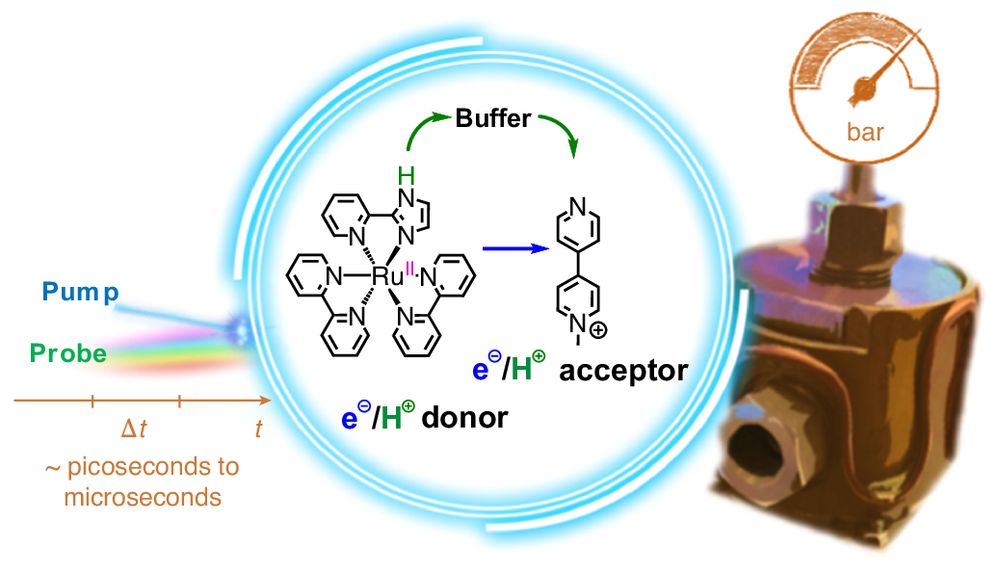
High-pressure pump–probe experiments reveal the mechanism of excited-state proton-coupled electron transfer and a shift from stepwise to concerted pathways
Nature Chemistry - Chemical energy conversion and storage rely on the selective movement of protons and electrons, thus understanding these processes is important for applications. Now experiments...
rdcu.be
Reposted by Enrique Soto
Daniel Reta
@danireta.bsky.social
· Jun 6

Radical Formation by Direct Single Electron Transfer between Nitrobenzene and Anionic Organo Bases
The presence of unpaired electrons, i.e., radicals, equips organic molecules with unique magnetic and reactivity properties. However, due to the reactive nature of radicals and the nontrivial chemistry required for their preparation, strict structural and electronic limitations are imposed on the available systems, limiting their potential applications. Thus, developing mechanisms that enable facile radical formation in simple reaction conditions, employing available and inexpensive reactants and applicable to general types of molecules, holds the key to capitalize on the extraordinary properties that radicals have to offer. Here, combining electron paramagnetic resonance spectroscopy and ab initio calculations, we uncover an unprecedented single electron transfer from multiple anionic organic bases (B–X+) to nitrobenzene [1], leading to the formation of stable nitrobenzenide radical ion-pair [1•–][X+] (X = Li, Na, K) and transient oxidized, radical bases B•. Our results establish nitroarenes as versatile radical precursors, providing a broadly applicable protocol for generating heteroatom-centered radicals and enabling radical transformations under mild conditions from inexpensive and readily available starting materials. Finally, we propose the [1]–[B–X+] couple as an unexplored platform with the potential to advance the field of frustrated radical pairs.
pubs.acs.org






















