Lack of basic rationale in epithelial-mesenchymal transition and its related concepts - Cell & Bioscience
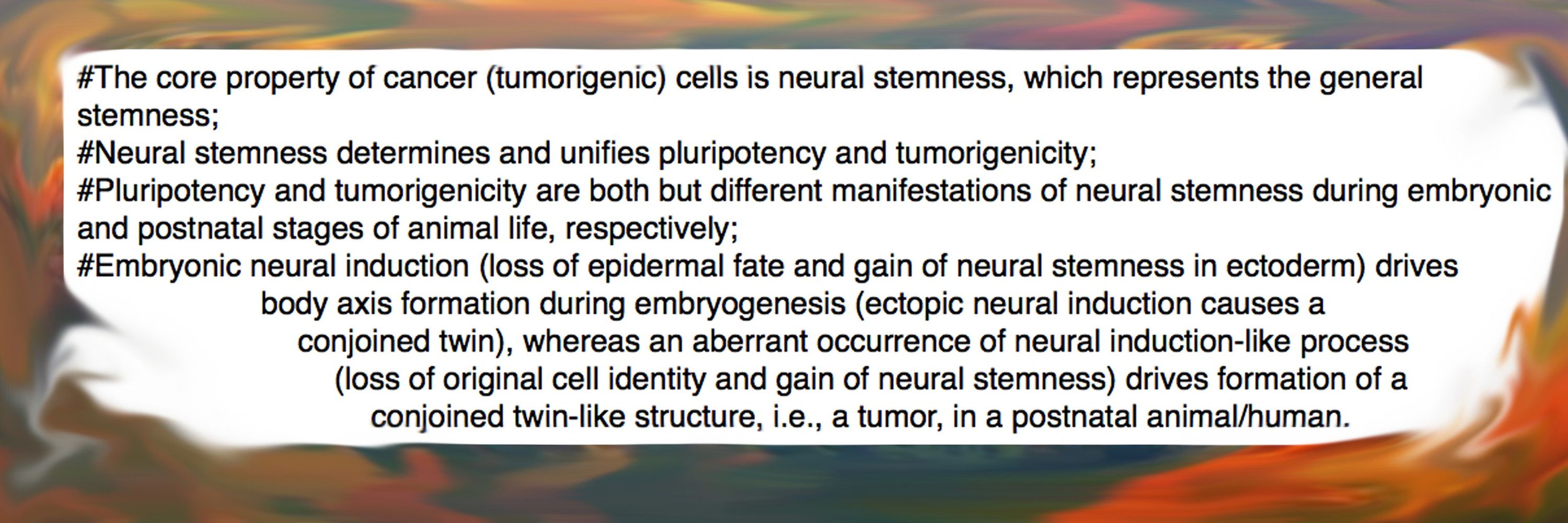
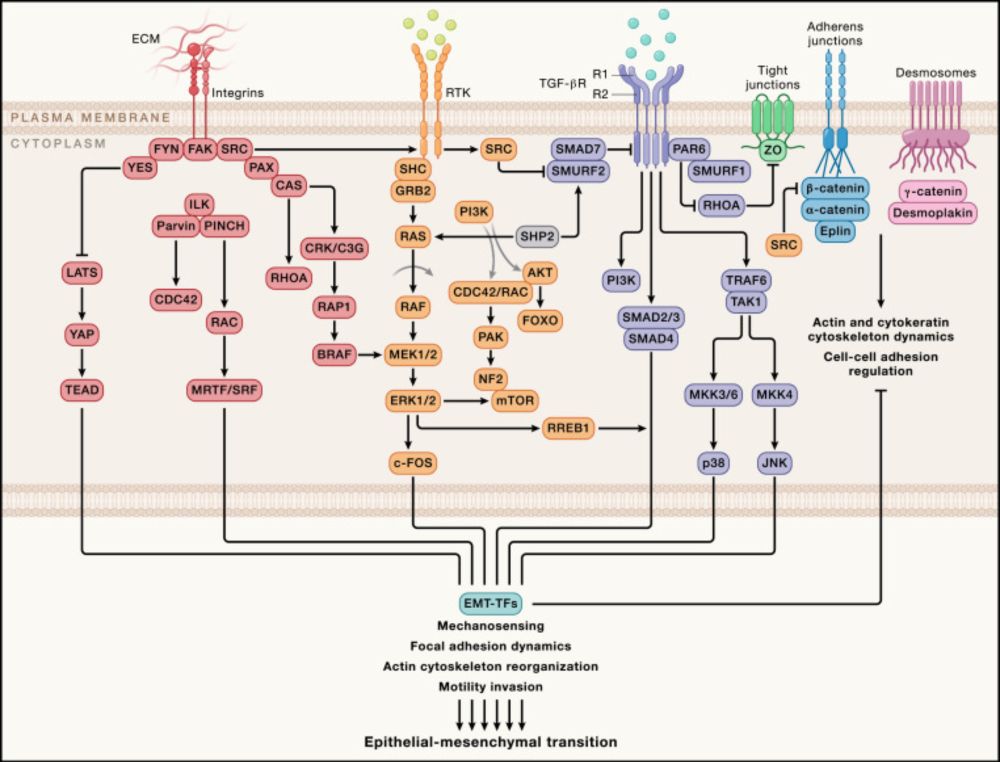
Epithelial–mesenchymal transition (EMT) is defined as a cellular process during which epithelial cells acquire mesenchymal phenotypes and behavior following the downregulation of epithelial features. EMT and its reversed process, the mesenchymal-epithelial transition (MET), and the special form of EMT, the endothelial-mesenchymal transition (EndMT), have been considered as mainstream concepts and general rules driving developmental and pathological processes, particularly cancer. However, discrepancies and disputes over EMT and EMT research have also grown over time. EMT is defined as transition between two cellular states, but it is unanimously agreed by EMT researchers that (1) neither the epithelial and mesenchymal states nor their regulatory networks have been clearly defined, (2) no EMT markers or factors can represent universally epithelial and mesenchymal states, and thus (3) EMT cannot be assessed on the basis of one or a few EMT markers. In contrast to definition and proposed roles of EMT, loss of epithelial feature does not cause mesenchymal phenotype, and EMT does not contribute to embryonic mesenchyme and neural crest formation, the key developmental events from which the EMT concept was derived. EMT and MET, represented by change in cell shapes or adhesiveness, or symbolized by EMT factors, are biased interpretation of the overall change in cellular property and regulatory networks during development and cancer progression. Moreover, EMT and MET are consequences rather than driving factors of developmental and pathological processes. The true meaning of EMT in some developmental and pathological processes, such as fibrosis, needs re-evaluation. EMT is believed to endow malignant features, such as migration, stemness, etc., to cancer cells. However, the core property of cancer (tumorigenic) cells is neural stemness, and the core EMT factors are components of the regulatory networks of neural stemness. Thus, EMT in cancer progression is misattribution of the roles of neural stemness to the unknown mesenchymal state. Similarly, neural crest EMT is misattribution of intrinsic property of neural crest cells to the unknown mesenchymal state. Lack of basic rationale in EMT and related concepts urges re-evaluation of their significance as general rules for understanding developmental and pathological processes, and re-evaluation of their significance in scientific research.