Posts
Media
Videos
Starter Packs
Nitzan Tal
@nitzantal.bsky.social
· Jul 19
Nitzan Tal
@nitzantal.bsky.social
· Jul 14
Nitzan Tal
@nitzantal.bsky.social
· Jul 14
Nitzan Tal
@nitzantal.bsky.social
· Jul 14
Nitzan Tal
@nitzantal.bsky.social
· Jul 14
Nitzan Tal
@nitzantal.bsky.social
· Jul 14
Nitzan Tal
@nitzantal.bsky.social
· Jul 13
Nitzan Tal
@nitzantal.bsky.social
· Jul 13
Nitzan Tal
@nitzantal.bsky.social
· Jul 13

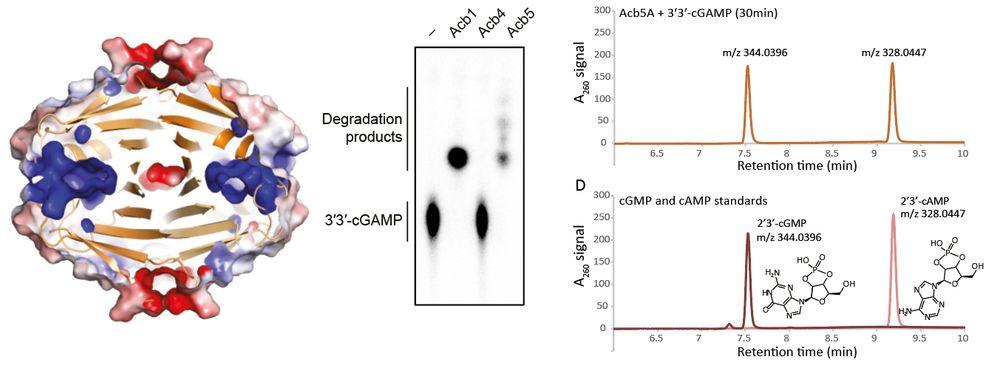
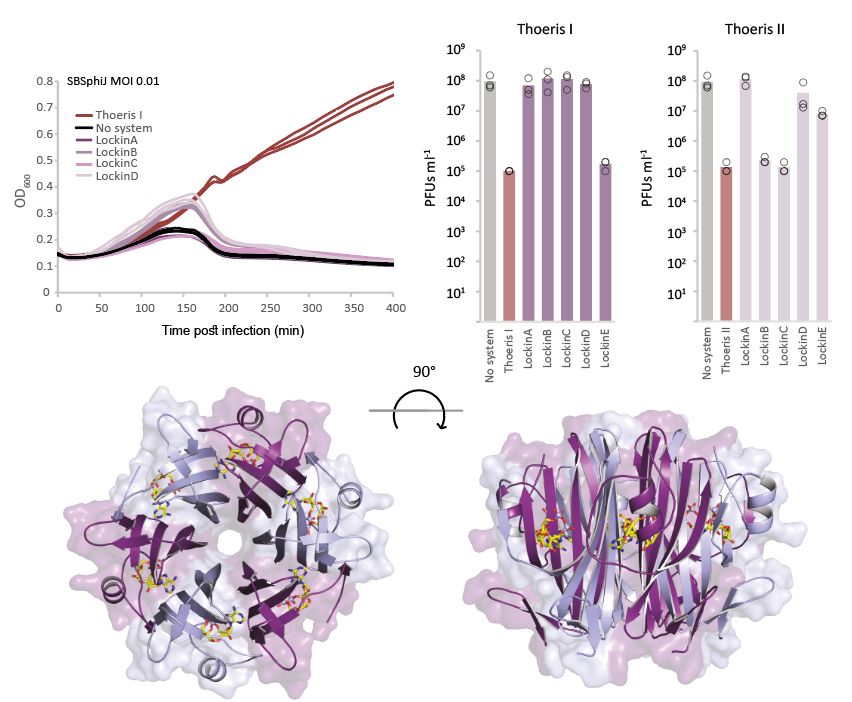
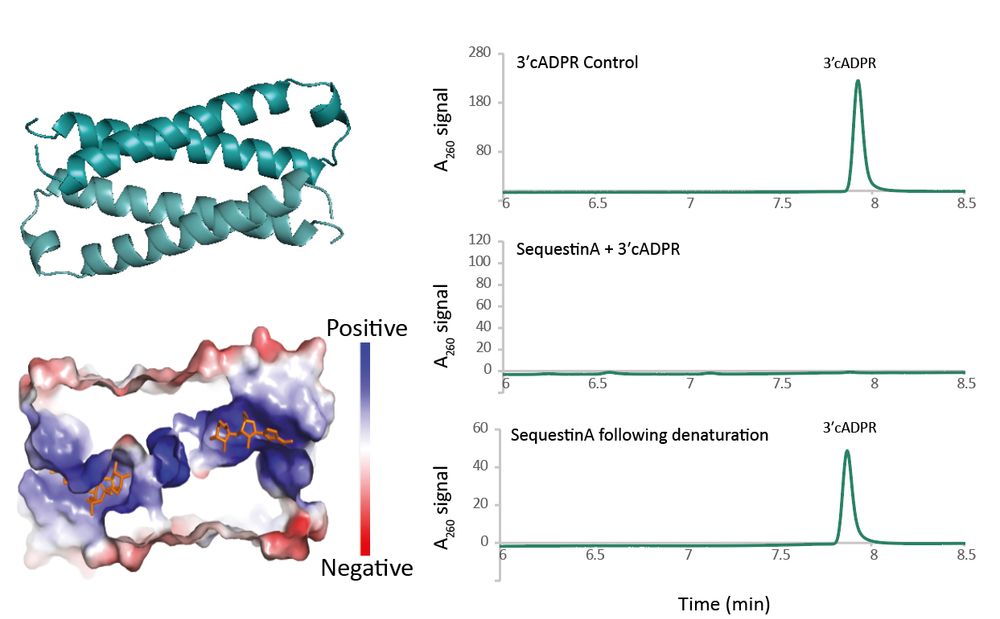
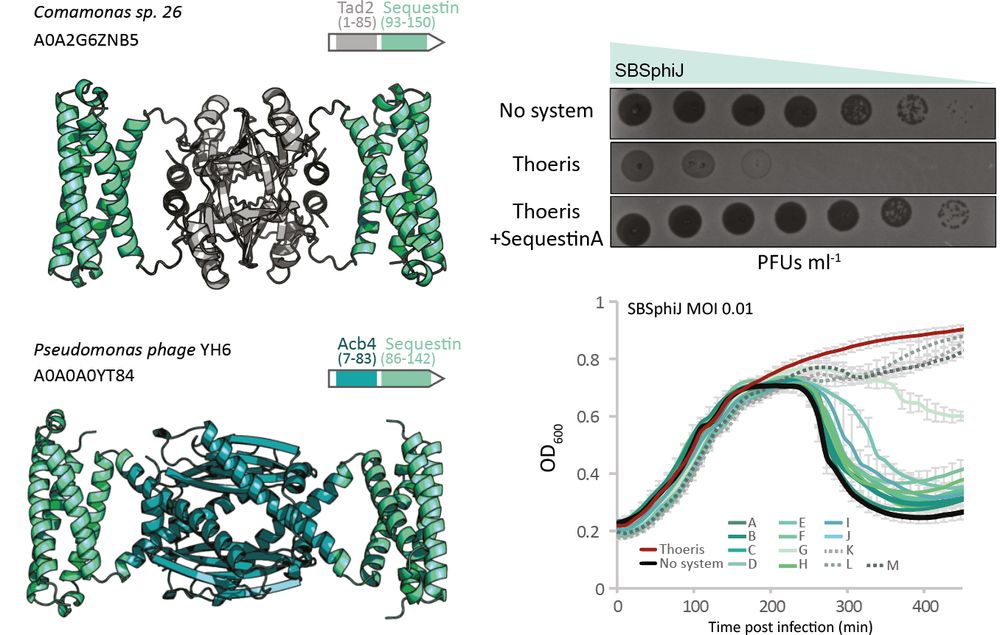
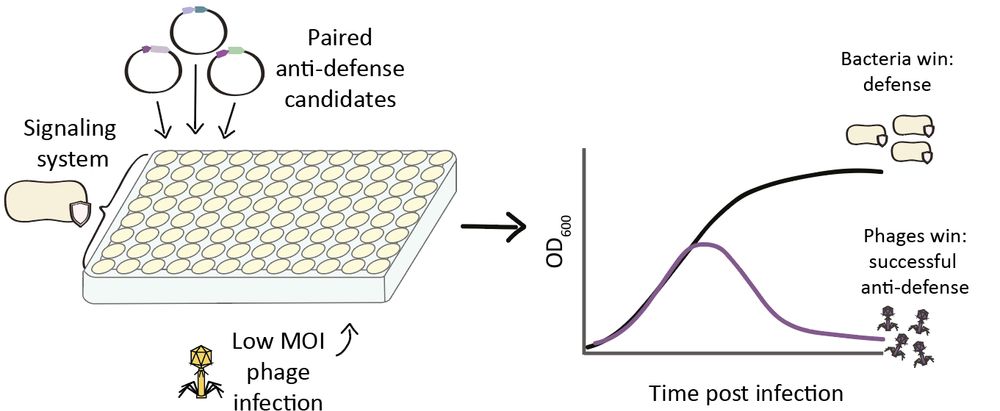
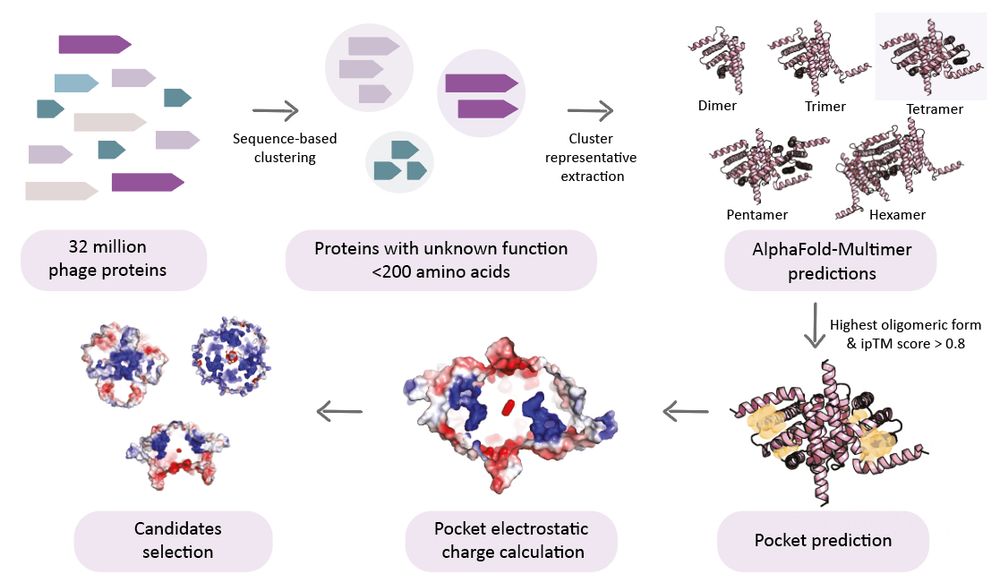
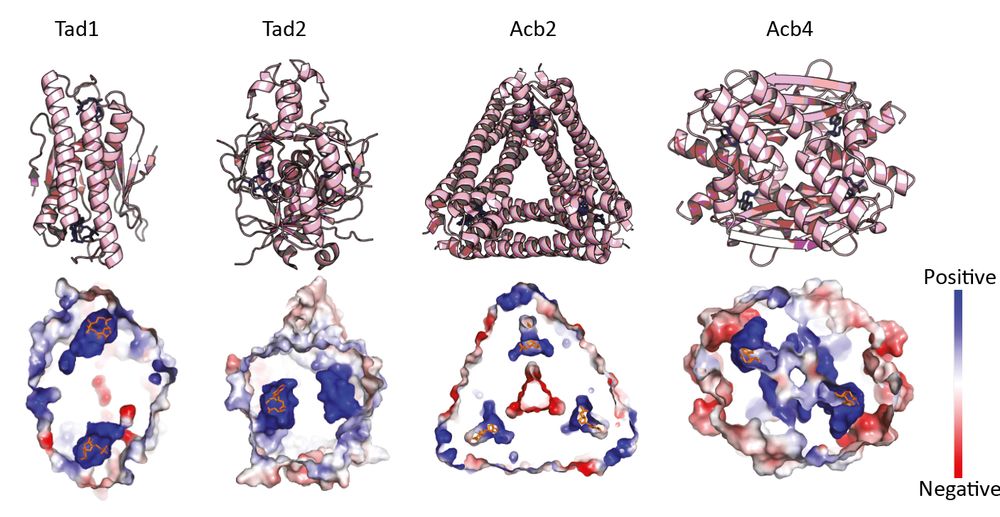
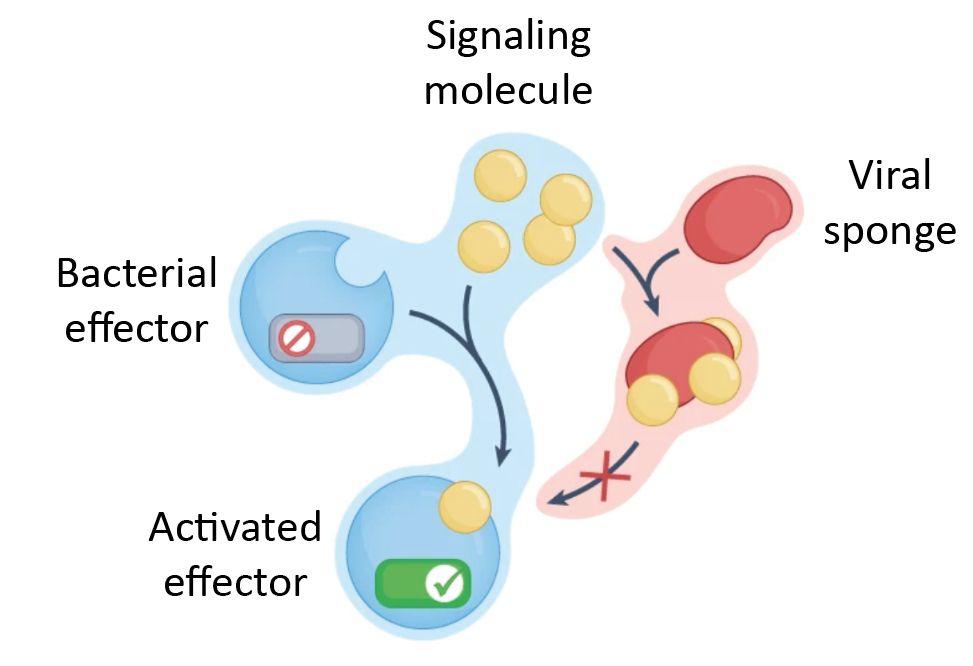
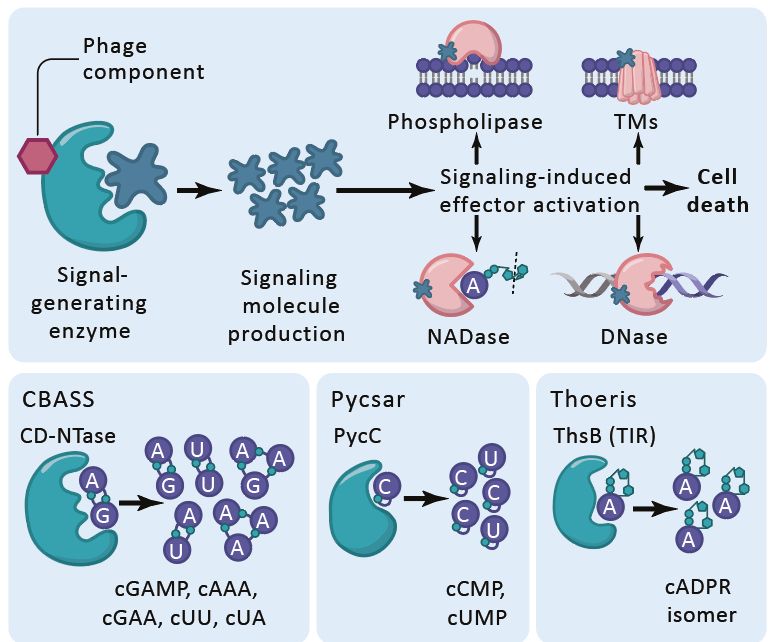
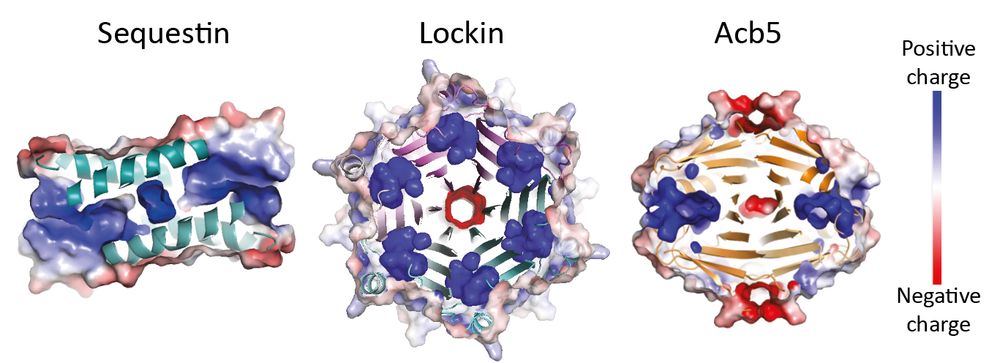
Structural modeling reveals viral proteins that manipulate host immune signaling
Immune pathways that use intracellular nucleotide signaling are common in animals, plants and bacteria. Viruses can inhibit nucleotide immune signaling by producing proteins that sequester or cleave t...
tinyurl.com
Nitzan Tal
@nitzantal.bsky.social
· Jul 13