Penn State Chemistry
@pennstatechemistry.bsky.social
260 followers
83 following
42 posts
The official account of the Department of Chemistry at Penn State University. Science Without Boundaries.
📍 University Park, PA
🖥️ https://science.psu.edu/chem
LinkedIn: https://www.linkedin.com/company/penn-state-chemistry
Posts
Media
Videos
Starter Packs
Pinned
Reposted by Penn State Chemistry
Jonathan Kuo
@jonathanlkuo.bsky.social
· Jun 23

Net Oxidative Addition of H2 to {MII}2+ (M = Pd, Pt) by Heterolysis and Protic Rebound
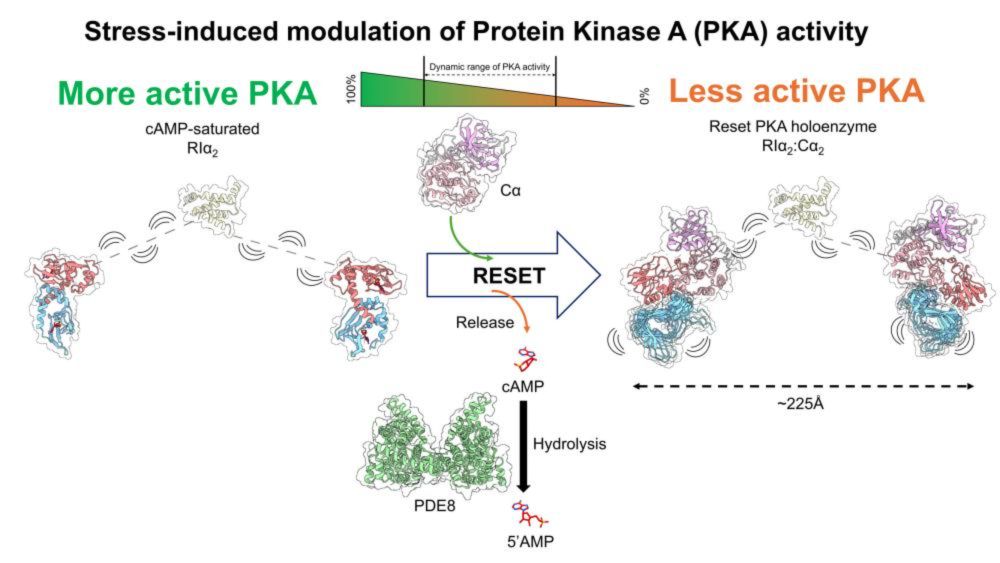
Electrophilic transition metal complexes like {MII(EtXantphos)2}2+ (MII = PdII, PtII) heterolyze H2 into a hydride-associated electrophile {H–MII(EtXantphos)2}+ and a proton, which typically associates to an added base (or basic ligand). For {H–MII(EtXantphos)2}+, the metal can be the most basic site in the system, which results in a product that is indistinguishable from oxidative addition {(H)2MIV(EtXantphos)2}2+. By considering the kinetics and thermodynamics of each elementary step – initial heterolysis, followed by a subsequent return of the heterolyzed proton – we suggest that oxidative addition products may be underrepresented tautomers in heterolytic pathways. The gained understanding was used to characterize the first (di)hydride of PdIV, generated by formal oxidative addition of H2 to PdII.
pubs.acs.org
Ayusman Sen
@ayusmansen.bsky.social
· Apr 23

Q&A: Microscopic robots may shape the future of health, tech and the environment | Penn State University
From microscopic robots that can carry and deliver drugs inside the human body to tiny particles that can detect and break down microplastics, researchers in an emerging field called active matter are...
www.psu.edu
Reposted by Penn State Chemistry
Kueyoung Kim
@kueyoungkim.bsky.social
· Apr 17
Reposted by Penn State Chemistry
Kueyoung Kim
@kueyoungkim.bsky.social
· Apr 17
Reposted by Penn State Chemistry
Reposted by Penn State Chemistry
Reposted by Penn State Chemistry
Ray Schaak
@rayschaak.bsky.social
· Mar 27
Reposted by Penn State Chemistry