Dave Leigh
@profdaveleigh.bsky.social
1.8K followers
250 following
31 posts
Royal Society Research Professor & Sir Samuel Hall Chair of Chemistry, University of Manchester, UK. European. molecules・ machines・ magic
Posts
Media
Videos
Starter Packs
Reposted by Dave Leigh
Reposted by Dave Leigh
Dave Leigh
@profdaveleigh.bsky.social
· Aug 29

Multiple Template Site Nitrogen Atom Deletions from Rotaxanes, Catenanes, and a Molecular Knot
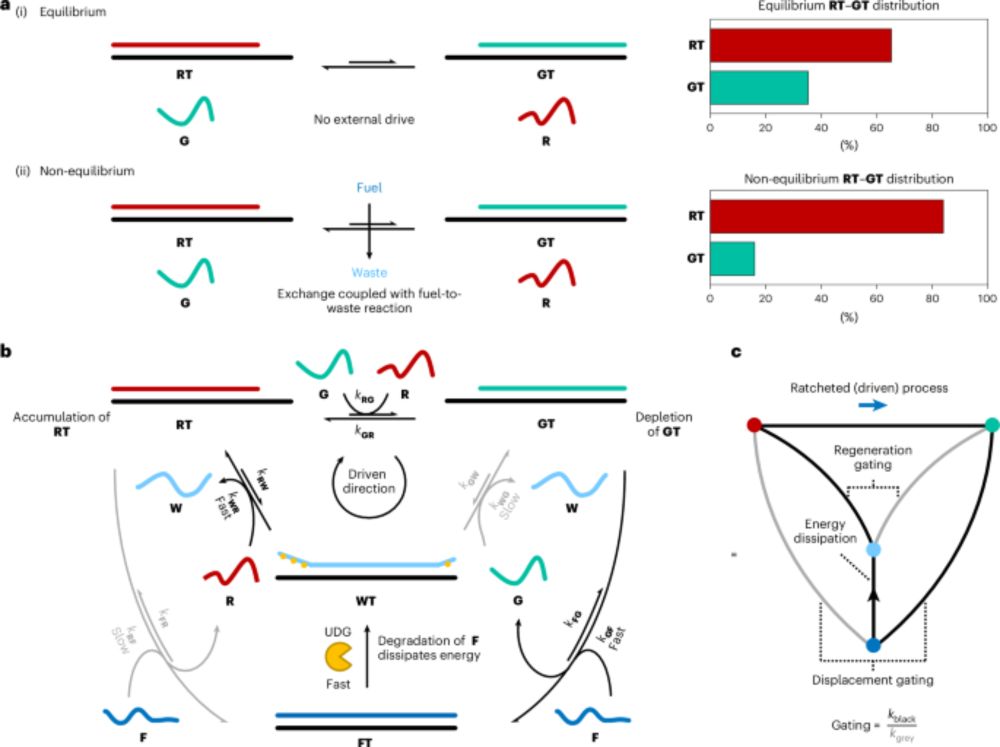
We report the deletion of nitrogen atoms from multiple template sites in rotaxanes, catenanes, and a molecular knot. Nitrogen extrusion from secondary amines in the backbone of the interlocked structures is achieved using O-diphenylphosphinylhydroxylamine (DPPH), forming carbon–carbon bonds while largely maintaining the integrity of the original mechanical bonding. We find that DPPH gives improved yields (up to 51%) for nitrogen atom deletions from template sites in rotaxanes compared to an anomeric amide nitrogen-deletion reagent and overcomes a major substrate limitation in that, using DPPH, only one of the substituents of the secondary amine in the rotaxane axle needs to be radical-stabilizing. Multiple template site nitrogen atom deletions were accomplished from a range of mechanically interlocked architectures, despite the potential for dethreading, unlinking, and/or strand uncrossing during each successive deletion event. Highlights include the deletion of two template sites from a doubly threaded [3]rotaxane (37% yield) and [3]catenane (45%), quadruple N-deletion of amines from both rings of a [2]catenane (33%), and six N-deletions from the six amine groups in a molecular trefoil knot (7%). The combination of skeletal editing with template synthesis provides a general strategy for synthesis that significantly increases the structural diversity of interlocked molecules that are potentially accessible.
pubs.acs.org
Dave Leigh
@profdaveleigh.bsky.social
· Aug 29
Dave Leigh
@profdaveleigh.bsky.social
· Aug 21
Reposted by Dave Leigh
Dave Leigh
@profdaveleigh.bsky.social
· Jul 12
Dave Leigh
@profdaveleigh.bsky.social
· Jul 9
Dave Leigh
@profdaveleigh.bsky.social
· Jul 9