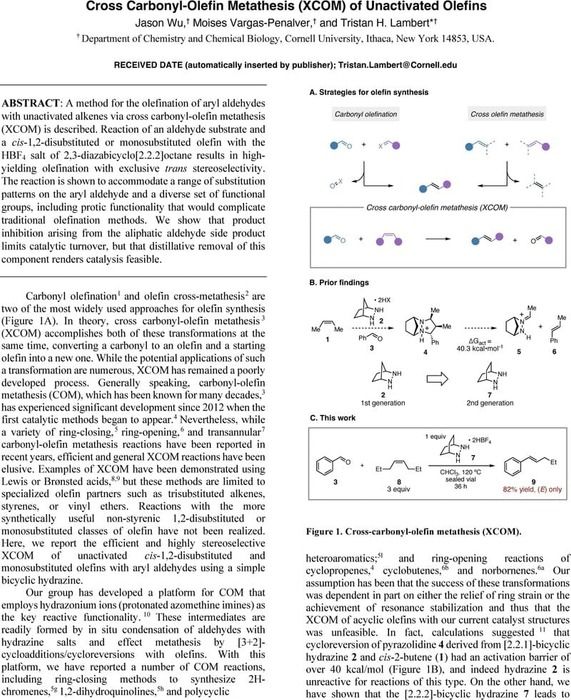
A Flexible and Affordable Self-Driving Laboratory for Automated Reaction Optimization
Self-driving laboratories (SDLs) have the potential to revolutionize chemical discovery and optimization, yet their widespread adoption remains limited by high costs, complex infrastructure, and limited accessibility. Here, we introduce RoboChem-Flex, a low-cost, modular self-driving laboratory platform designed to democratize autonomous chemical experimentation. The system combines customizable, in-house-built hardware with a flexible Python-based software framework that integrates real-time device control and advanced Bayesian optimization strategies, including multi-objective and transfer learning workflows. RoboChem-Flex supports both fully autonomous closed-loop operation and human-in-the-loop configurations, enabling seamless integration with shared analytical equipment and minimizing entry barriers. We validate the versatility of the platform across six diverse case studies, including photocatalysis, biocatalysis, thermal cross-couplings, and enantioselective catalysis, spanning both single and multi-objective optimizations. Through these campaigns, we demonstrate RoboChem-Flex’s ability to navigate large, complex chemical spaces, autonomously identify scalable high-performance reaction conditions, and flexibly adapt to a variety of analytical setups. By providing an affordable, scalable, and open platform, RoboChem-Flex offers a tangible step toward making SDLs accessible to resource-limited laboratories, fostering broader participation in automated chemical research.