YanFeng Zhang
@yanfengzhang.bsky.social
71 followers
160 following
20 posts
Neuroscience, Learning, Memory, Basal Ganglia, Lecturer@University of Exeter
Posts
Media
Videos
Starter Packs
YanFeng Zhang
@yanfengzhang.bsky.social
· Jun 19
YanFeng Zhang
@yanfengzhang.bsky.social
· Mar 18
Nature Neuroscience
@natneuro.nature.com
· Mar 18

An axonal brake on striatal dopamine output by cholinergic interneurons - Nature Neuroscience
Cholinergic interneurons act at nicotinic receptors to depress dopamine release, interrupting its relationship to dopamine neuron firing and supporting an inverse scaling of dopamine release according...
www.nature.com
YanFeng Zhang
@yanfengzhang.bsky.social
· Mar 17
YanFeng Zhang
@yanfengzhang.bsky.social
· Mar 17
YanFeng Zhang
@yanfengzhang.bsky.social
· Mar 17
YanFeng Zhang
@yanfengzhang.bsky.social
· Mar 17
YanFeng Zhang
@yanfengzhang.bsky.social
· Mar 17
YanFeng Zhang
@yanfengzhang.bsky.social
· Mar 17
YanFeng Zhang
@yanfengzhang.bsky.social
· Mar 17
YanFeng Zhang
@yanfengzhang.bsky.social
· Mar 17
YanFeng Zhang
@yanfengzhang.bsky.social
· Mar 17
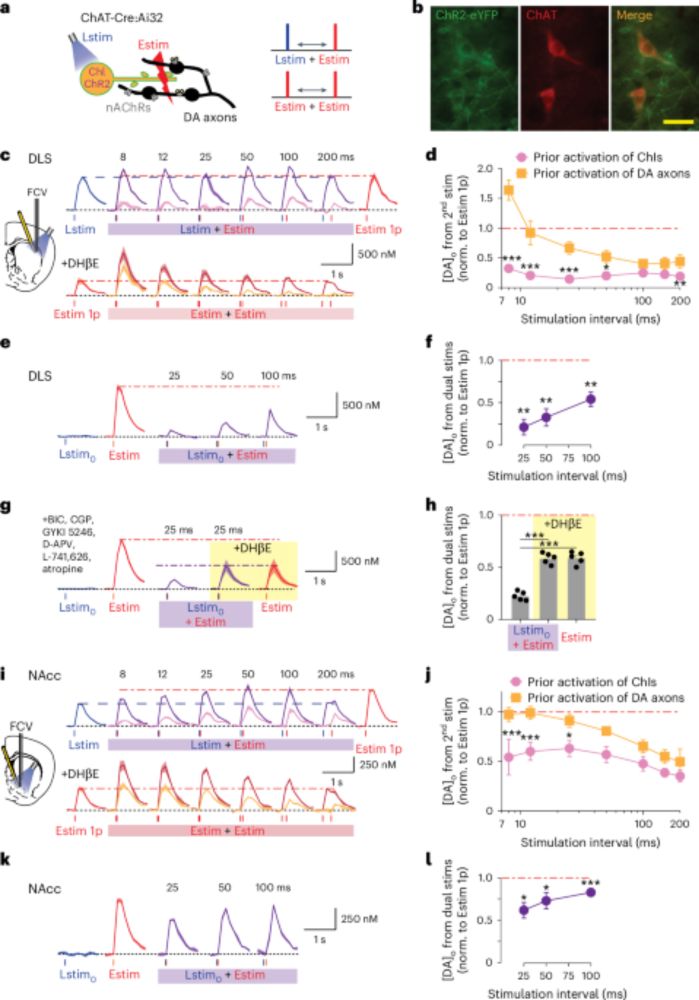
An axonal brake on striatal dopamine output by cholinergic interneurons - Nature Neuroscience
Cholinergic interneurons act at nicotinic receptors to depress dopamine release, interrupting its relationship to dopamine neuron firing and supporting an inverse scaling of dopamine release according...
www.nature.com
YanFeng Zhang
@yanfengzhang.bsky.social
· Mar 17
YanFeng Zhang
@yanfengzhang.bsky.social
· Mar 17
YanFeng Zhang
@yanfengzhang.bsky.social
· Mar 17
YanFeng Zhang
@yanfengzhang.bsky.social
· Mar 17
YanFeng Zhang
@yanfengzhang.bsky.social
· Mar 17
YanFeng Zhang
@yanfengzhang.bsky.social
· Mar 17
YanFeng Zhang
@yanfengzhang.bsky.social
· Mar 17
YanFeng Zhang
@yanfengzhang.bsky.social
· Mar 17