Kozlowski Lab
@kozlowskigroup.bsky.social
450 followers
28 following
35 posts
Organic chemistry research group at UPenn, interested in reactions and molecules from all perspectives, on the look out for interesting chemistry challenges.
Posts
Media
Videos
Starter Packs
Reposted by Kozlowski Lab
Kozlowski Lab
@kozlowskigroup.bsky.social
· Jun 30

Synthesis and Blending of Two Poly(ethylene-co-vinyl alcohol) Polymers with Mixed 1,2-Diol Stereochemistry
Parallel pathways for the postpolymerization modification of double bonds in a polycyclooctene (PCOE) backbone generate vicinal 1,2-diol-containing polymers with mixed but opposite stereochemistry, depending on the trans:cis ratio of the C═C in PCOE. Beginning from the same batch of PCOE, epoxidation and subsequent ring-opening with sulfuric acid and water produce a polymer with the majority erythro diols, whereas an osmium-catalyzed dihydroxylation results in diols in the majority threo orientations. These postpolymerization modification approaches enable access to previously unexplored polymers with a mixture of erythro and threo diols, offering tunable diol stereochemistry to tailor material properties. The majority erythro diols lead to hexagonal crystallites with higher melting temperatures and overall crystallinity when compared to the majority threo diols that form monoclinic crystallites. When blended, the two diastereomers phase separate as evidenced by distinct melting endotherms and crystal structures corresponding to the two component polymers, suggesting a route to tune the barrier or mechanical properties. This investigation synthesized polymers with mixed stereochemical diols and elucidated the thermal and morphological properties of regioregular linear poly(ethylene-co-vinyl alcohols) and their blends.
pubs.acs.org
Reposted by Kozlowski Lab
Penn Chemistry
@pennchemistry.bsky.social
· Jun 16
Reposted by Kozlowski Lab
Weix Group
@weixgroup.bsky.social
· Mar 7
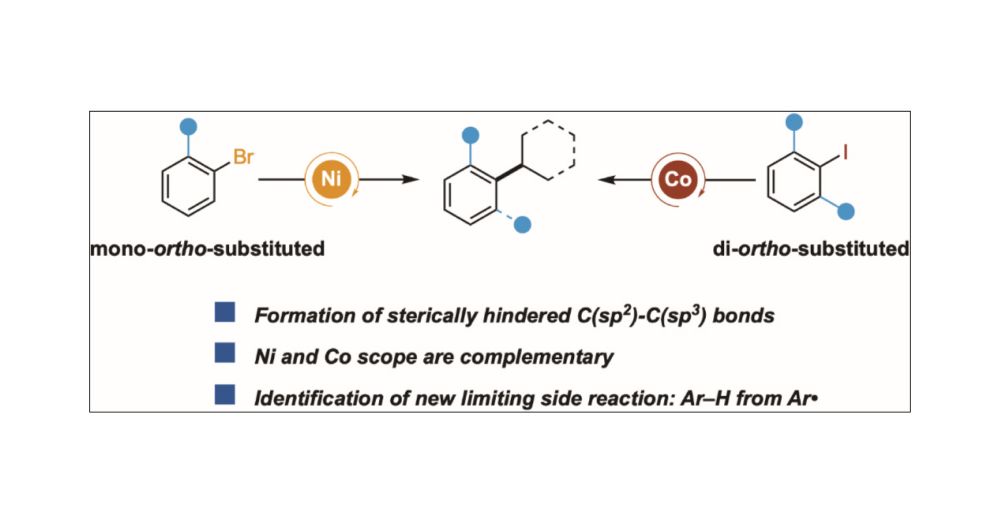
Cross-Electrophile Coupling to Form Sterically Hindered C(sp2)–C(sp3) Bonds: Ni and Co Afford Complementary Reactivity
The formation of sterically hindered C(sp2)–C(sp3) bonds could be a useful synthetic tool but has been understudied in cross-electrophile coupling. Here, we report two methods that couple secondary alkyl bromides with aryl halides that contain sterically hindered C–X bonds: 1) ortho-substituted aryl bromides with nickel catalysts and 2) di-ortho-substituted aryl iodides with cobalt catalysts. Stoichiometric experiments and deuterium labeling studies show that 1) [Co] is better than [Ni] for oxidative addition of di-ortho-substituted Ar–I and 2) [Co] is better than [Ni] for radical capture/reductive elimination steps with di-ortho-substituted arenes. For both metals, Ar–H side products observed in reactions with low-yielding di-ortho-substituted aryl iodides appear to arise from Ar• formation and hydrogen-atom transfer from the solvent. While the origins of the differences in scope are not yet understood, these studies demonstrate a previously unknown complementarity between nickel and cobalt in cross-electrophile coupling.
doi.org
Weix Group
@weixgroup.bsky.social
· Mar 7

Cross-Electrophile Coupling to Form Sterically Hindered C(sp2)–C(sp3) Bonds: Ni and Co Afford Complementary Reactivity
The formation of sterically hindered C(sp2)–C(sp3) bonds could be a useful synthetic tool but has been understudied in cross-electrophile coupling. Here, we report two methods that couple secondary alkyl bromides with aryl halides that contain sterically hindered C–X bonds: 1) ortho-substituted aryl bromides with nickel catalysts and 2) di-ortho-substituted aryl iodides with cobalt catalysts. Stoichiometric experiments and deuterium labeling studies show that 1) [Co] is better than [Ni] for oxidative addition of di-ortho-substituted Ar–I and 2) [Co] is better than [Ni] for radical capture/reductive elimination steps with di-ortho-substituted arenes. For both metals, Ar–H side products observed in reactions with low-yielding di-ortho-substituted aryl iodides appear to arise from Ar• formation and hydrogen-atom transfer from the solvent. While the origins of the differences in scope are not yet understood, these studies demonstrate a previously unknown complementarity between nickel and cobalt in cross-electrophile coupling.
doi.org
Reposted by Kozlowski Lab
Perry Group
@perrychem.bsky.social
· Mar 5



































