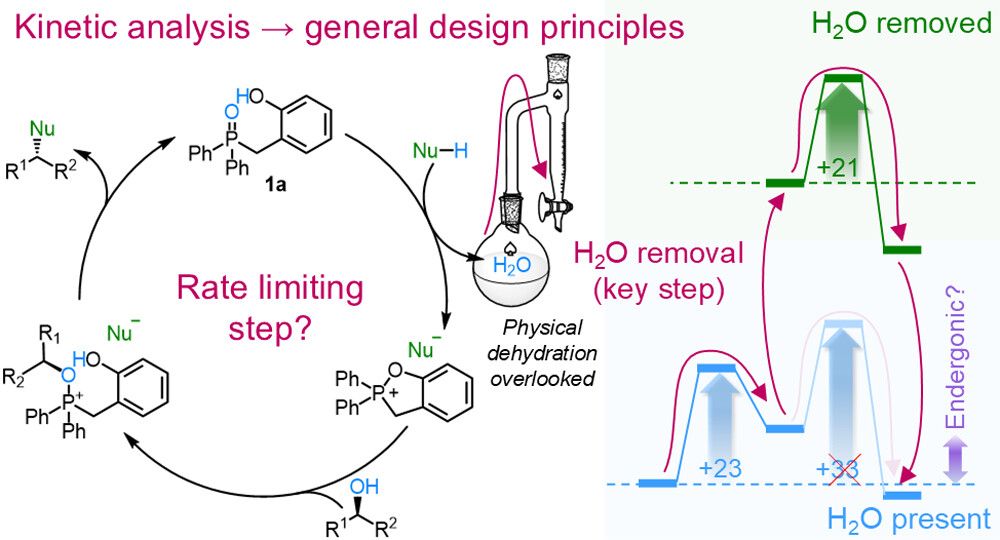
Congrats to Ethan Lim and everyone involved!
www.nature.com/articles/s44...
#Chemistry #Collaboration #AcademicIndustry #compchem

Congrats to Ethan Lim and everyone involved!
www.nature.com/articles/s44...
#Chemistry #Collaboration #AcademicIndustry #compchem

They left Oldham after WWII, when jobs were in short supply for unskilled workers.
I return a few years later to give the TY Shen Lecture University of Manchester with my kind host @profdaveleigh.bsky.social

They left Oldham after WWII, when jobs were in short supply for unskilled workers.
I return a few years later to give the TY Shen Lecture University of Manchester with my kind host @profdaveleigh.bsky.social
@iscr-rennes.bsky.social

@iscr-rennes.bsky.social
www.nature.com/articles/s41...
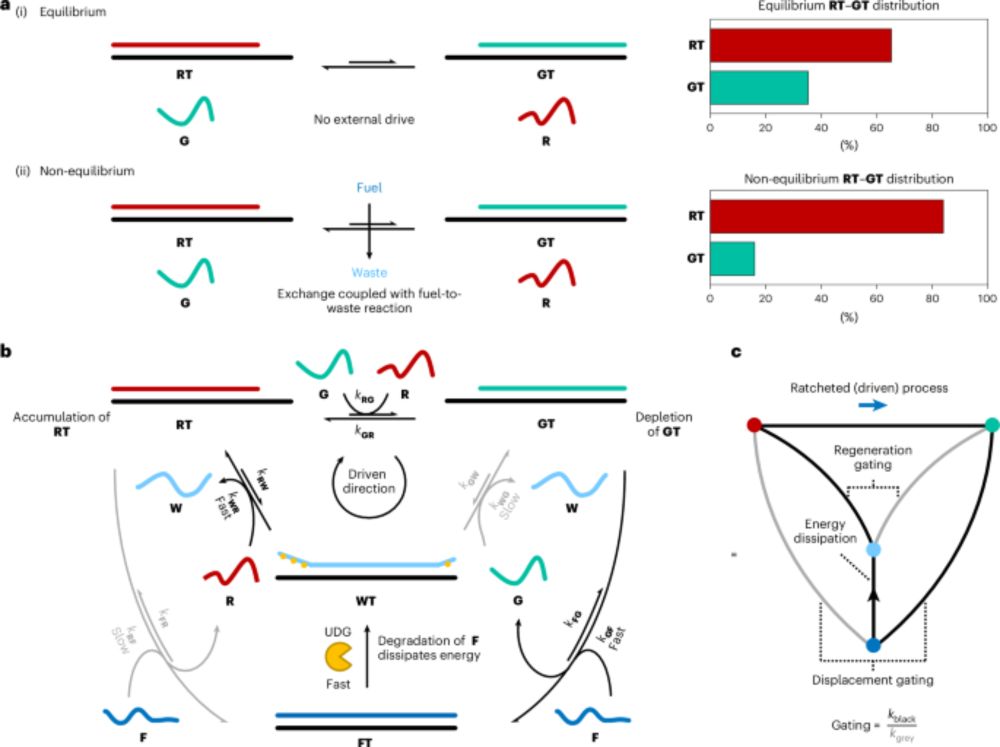
www.nature.com/articles/s41...
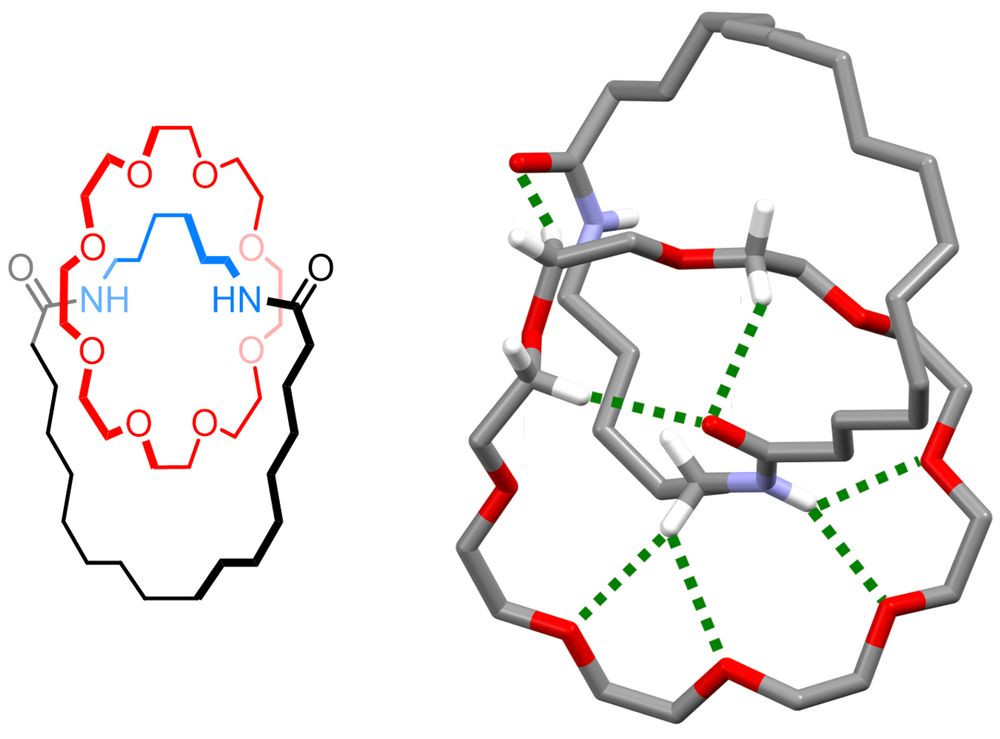
pubs.acs.org/doi/10.1021/...
@profdaveleigh.bsky.social @stefanborsley.bsky.social
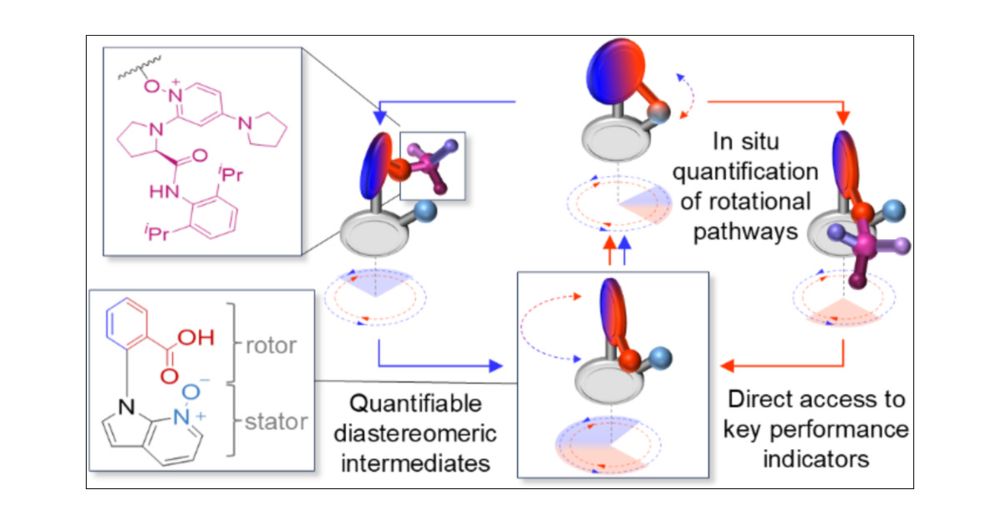
pubs.acs.org/doi/10.1021/...
@profdaveleigh.bsky.social @stefanborsley.bsky.social








It's not just budgets but research, institutions, expertise, and training the next generation.
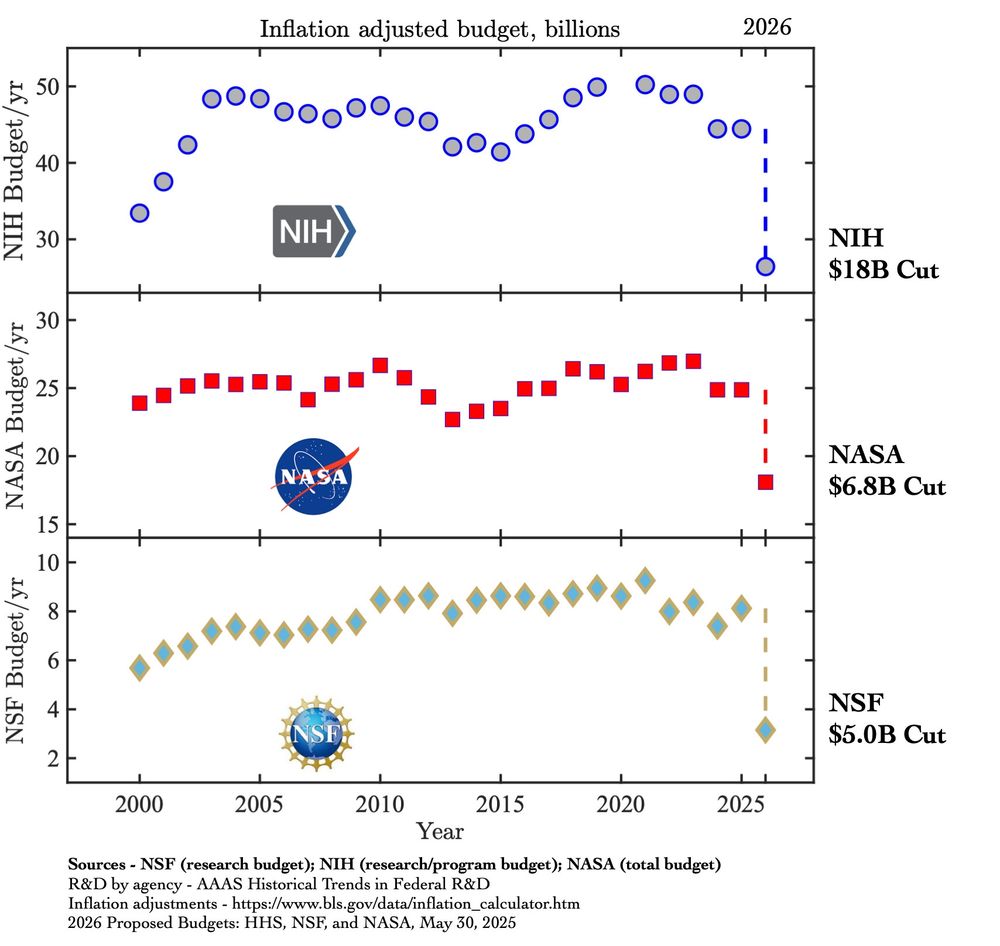
It's not just budgets but research, institutions, expertise, and training the next generation.