
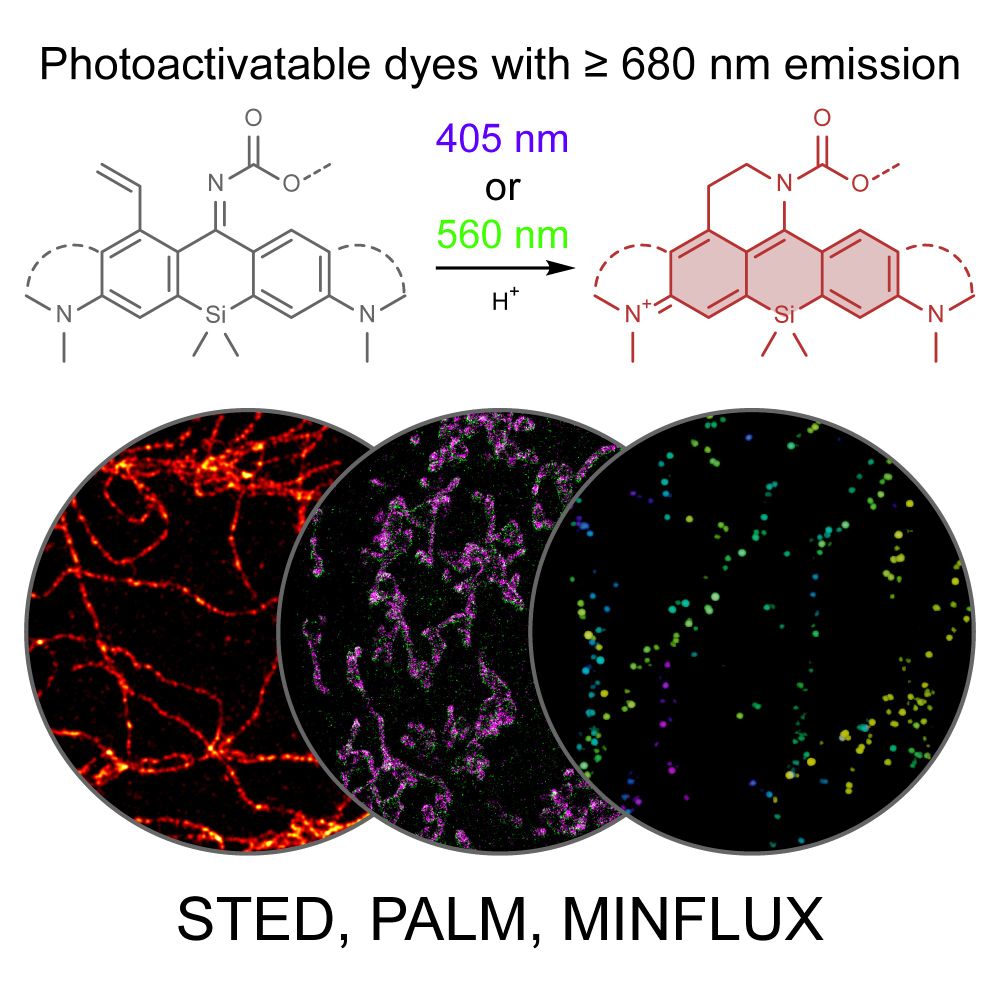
www.biorxiv.org/content/10.1...
We’re looking for an organic chemist to help develop novel fluorogenic HaloTag ligands for deep-tissue imaging. You’ll contribute to molecular design and run in silico, in vitro, and microscopy-based assays to quantify fluorophore performance.
We’re looking for an organic chemist to help develop novel fluorogenic HaloTag ligands for deep-tissue imaging. You’ll contribute to molecular design and run in silico, in vitro, and microscopy-based assays to quantify fluorophore performance.
#Neuroscience
#Neuroscience
www.biorxiv.org/content/10.1...

www.biorxiv.org/content/10.1...

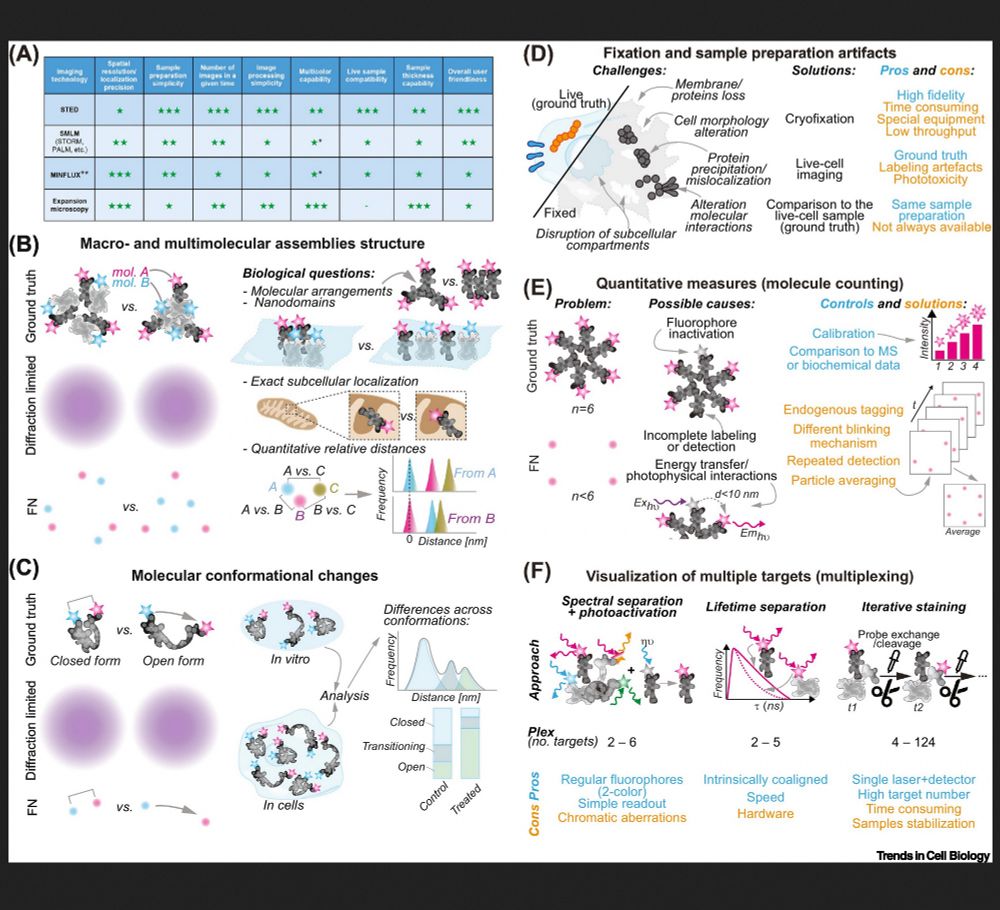
Check out our new review in Trends in Cell Biology:
www.sciencedirect.com/science/arti...
Thanks and kudos to all authors: Elisa DʼEste, Gražvydas Lukinavičius, Felipe Opazo and @rlicolnchemist.bsky.social
Check out our new review in Trends in Cell Biology:
www.sciencedirect.com/science/arti...
Thanks and kudos to all authors: Elisa DʼEste, Gražvydas Lukinavičius, Felipe Opazo and @rlicolnchemist.bsky.social

