
trialsjournal.biomedcentral.com/articles/10....
trialsjournal.biomedcentral.com/articles/10....

www.findaphd.com/phds/project...

www.findaphd.com/phds/project...

Help improve clinical trials by sharing your thoughts on health data collection.
@anniestats.bsky.social a PhD researcher at Imperial has designed this survey with the help of her public involvement groups.
🔗 imperial.eu.qualtrics.com/jfe/form/SV_...
#PPI #HealthEquity #ResearchVoices
Help improve clinical trials by sharing your thoughts on health data collection.
@anniestats.bsky.social a PhD researcher at Imperial has designed this survey with the help of her public involvement groups.
🔗 imperial.eu.qualtrics.com/jfe/form/SV_...
#PPI #HealthEquity #ResearchVoices


bmjopen.bmj.com/content/15/8...

bmjopen.bmj.com/content/15/8...
A new review of protocols led by Timothy Clark shows many incorrectly defined estimand attributes. See the top areas for improvement & full results here:
trialsjournal.biomedcentral.com/articles/10.... #Trials

A new review of protocols led by Timothy Clark shows many incorrectly defined estimand attributes. See the top areas for improvement & full results here:
trialsjournal.biomedcentral.com/articles/10.... #Trials
Imperial Clinical Trials Unit are looking for an ambitious senior statistician with experience in clinical trials & design & analysis of adaptive trials to lead a research programme in antimicrobial resistance & data-enabled trials.
Full details: www.imperial.ac.uk/jobs/search-...


This can happen more often than you think, and can have a dramatic impact on trial results (e.g. a false-positive rate of almost 90%)
arxiv.org/abs/2506.22610 @timpmorris.bsky.social

This can happen more often than you think, and can have a dramatic impact on trial results (e.g. a false-positive rate of almost 90%)
arxiv.org/abs/2506.22610 @timpmorris.bsky.social


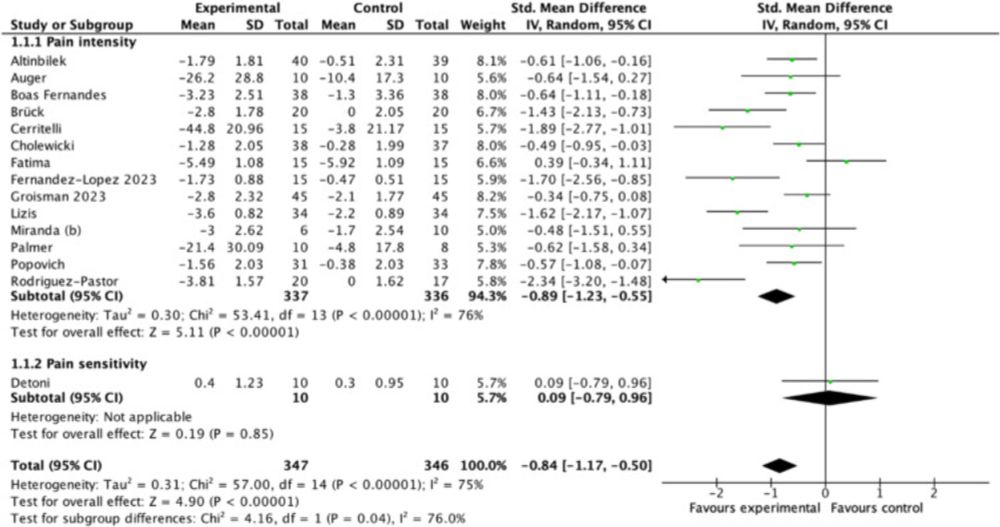
New blog post introducing **Retrieved Dropout Reference-Based Centred Multiple Imputation**

New blog post introducing **Retrieved Dropout Reference-Based Centred Multiple Imputation**
@jclinepi.bsky.social
www.jclinepi.com/article/S089...
@jclinepi.bsky.social
www.jclinepi.com/article/S089...
Day 1 had a range of brilliant speakers and overall was a very inspiring day of clinical trials! Can’t wait for the next one
Day 1 had a range of brilliant speakers and overall was a very inspiring day of clinical trials! Can’t wait for the next one
shorturl.at/IioXW
Closing 5th May

shorturl.at/IioXW
Closing 5th May



