Many thanks to my amazing colleagues Ann-Sophie Paschke, Bence Béla Botlik, and former students Francesco Felician and Nima Nasiri!
www.science.org/doi/10.1126/...




Ann-Sophie Paschke, Nima Nasiri, Bence Botlik
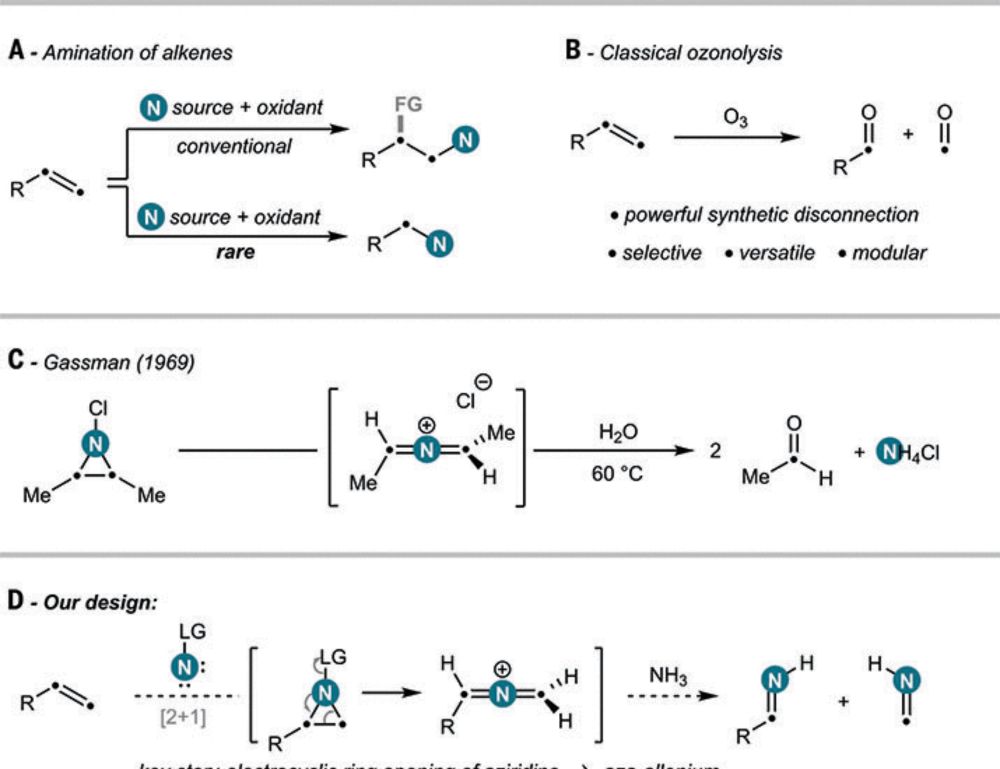
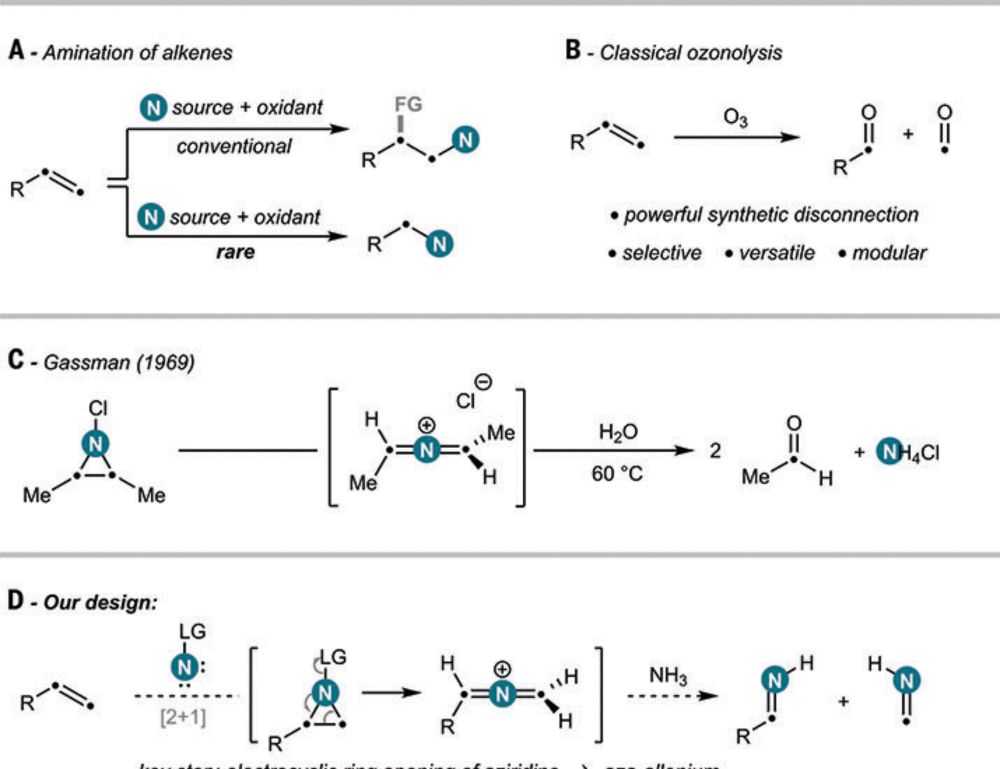
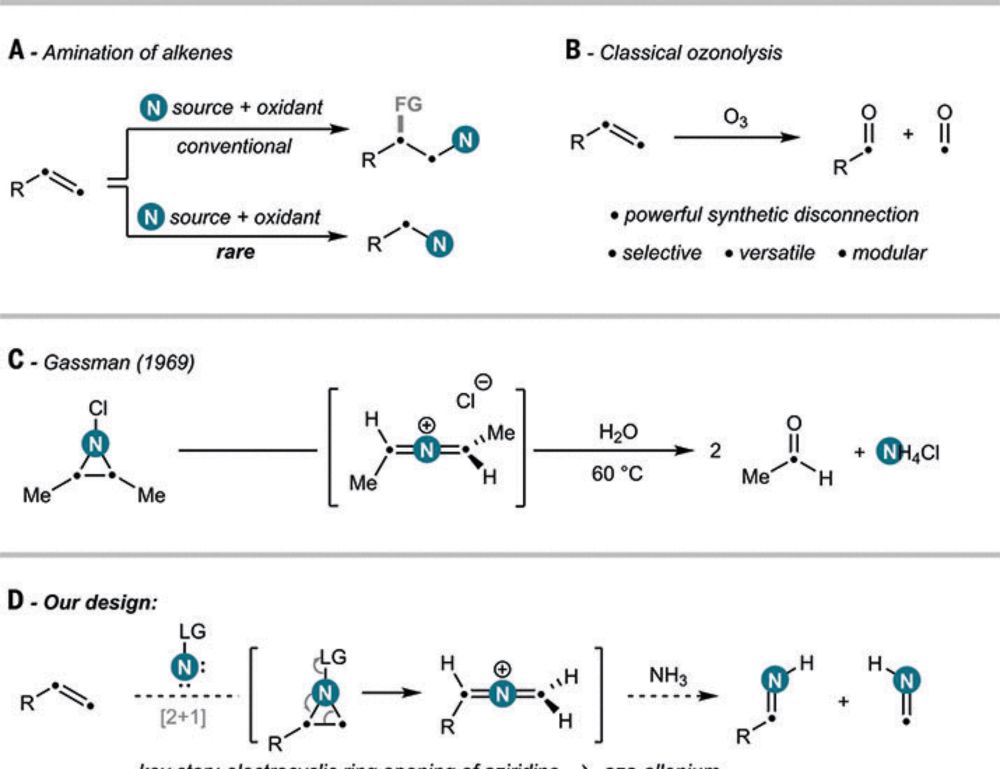
and Francesco Felician for this masterpiece! Oxidative amination by nitrogen atom insertion into carbon-carbon double bonds in Science, @ethzurich.bsky.social.
www.science.org/doi/10.1126/...
Ann-Sophie Paschke, Nima Nasiri, Bence Botlik
and Francesco Felician for this masterpiece! Oxidative amination by nitrogen atom insertion into carbon-carbon double bonds in Science, @ethzurich.bsky.social.
www.science.org/doi/10.1126/...
Many thanks to my amazing colleagues Ann-Sophie Paschke, Bence Béla Botlik, and former students Francesco Felician and Nima Nasiri!
www.science.org/doi/10.1126/...

Many thanks to my amazing colleagues Ann-Sophie Paschke, Bence Béla Botlik, and former students Francesco Felician and Nima Nasiri!
www.science.org/doi/10.1126/...
www.science.org/doi/10.1126/...
www.science.org/doi/10.1126/...
Now out on arXiv: arxiv.org/abs/2502.18966
A short explanation thread 👇

Now out on arXiv: arxiv.org/abs/2502.18966
A short explanation thread 👇

