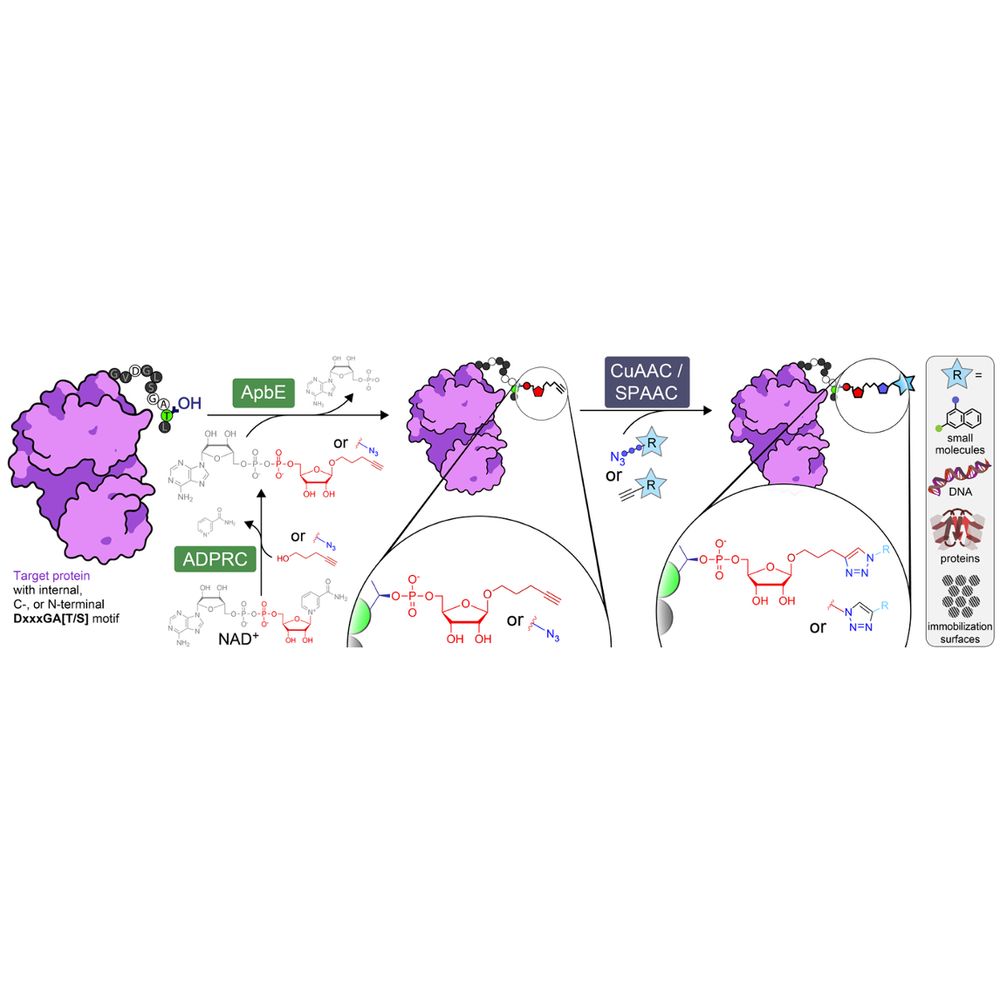
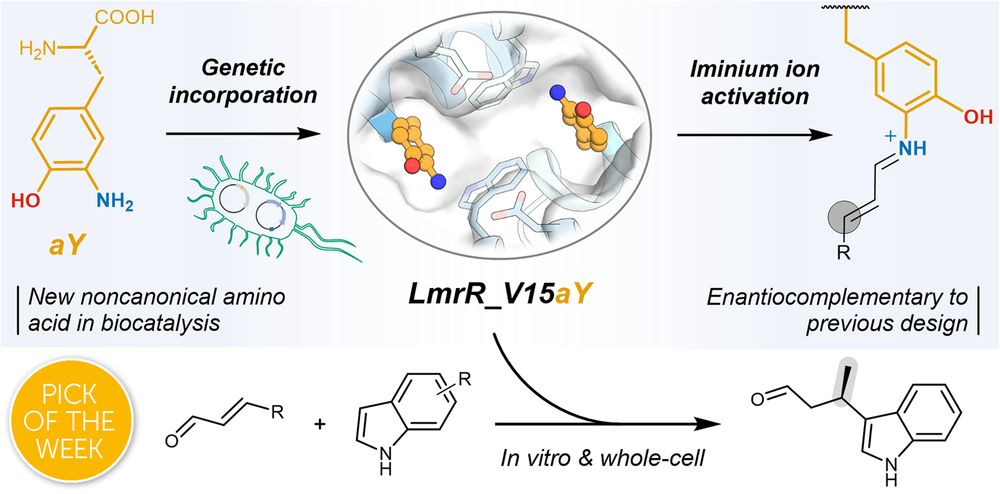
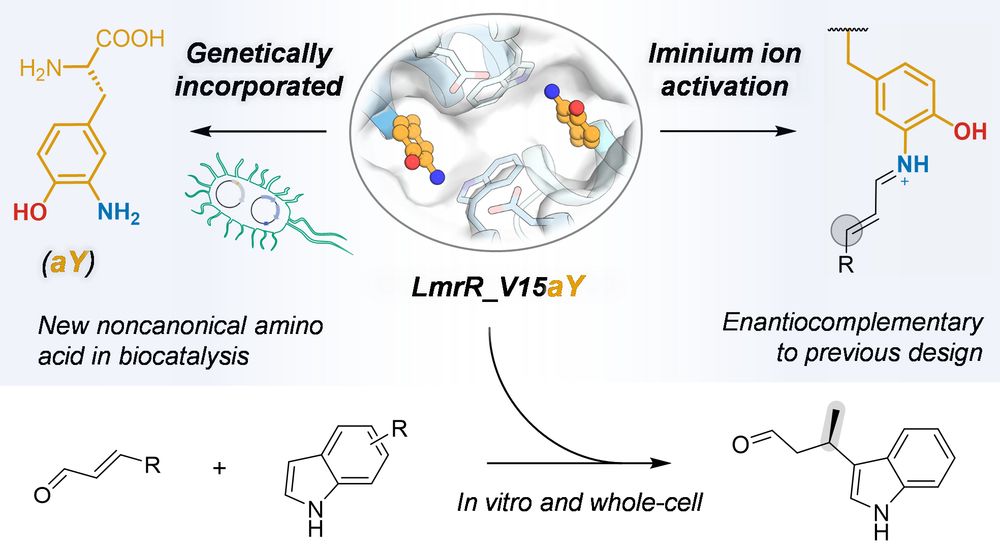
Bart Brouwer
@brouwerb.bsky.social
43 followers
61 following
13 posts
Postdoc | Artificial Enzyme Design | Drienovská lab | VU Amsterdam 🇳🇱
Previously: PhD | Roelfes Group | RuG
Posts
Media
Videos
Starter Packs
Reposted by Bart Brouwer
Bart Brouwer
@brouwerb.bsky.social
· Apr 23
Bart Brouwer
@brouwerb.bsky.social
· Apr 23
Bart Brouwer
@brouwerb.bsky.social
· Apr 23
Bart Brouwer
@brouwerb.bsky.social
· Apr 23
Bart Brouwer
@brouwerb.bsky.social
· Apr 23
Bart Brouwer
@brouwerb.bsky.social
· Apr 23
Bart Brouwer
@brouwerb.bsky.social
· Apr 23
Reposted by Bart Brouwer
Bart Brouwer
@brouwerb.bsky.social
· Feb 11
Bart Brouwer
@brouwerb.bsky.social
· Feb 11
Bart Brouwer
@brouwerb.bsky.social
· Feb 11
Bart Brouwer
@brouwerb.bsky.social
· Feb 11
Bart Brouwer
@brouwerb.bsky.social
· Feb 11