Stellar lineup, Keynotes from Tobias Meyer and Jin Zhang
Join us in Nashville!
tinyurl.com/bd9wmyve

Stellar lineup, Keynotes from Tobias Meyer and Jin Zhang
Join us in Nashville!
tinyurl.com/bd9wmyve




1) EML4-ALK condensation is biphasic (highest at mid levels), but signaling increases monotonically w expression. Thus the strongest signaling is in cells with *no* condensates.

1) EML4-ALK condensation is biphasic (highest at mid levels), but signaling increases monotonically w expression. Thus the strongest signaling is in cells with *no* condensates.

www.biorxiv.org/content/10.6...
👇

www.biorxiv.org/content/10.6...
👇


See concurrent preprint for more surprises on the role of condensates in fusions.
www.biorxiv.org/content/10.6...

See concurrent preprint for more surprises on the role of condensates in fusions.
www.biorxiv.org/content/10.6...

www.nature.com/articles/s41....
Do other fusions also suppress EGFR? Also EML4-ALK formed condensates -- are condensates important?

www.nature.com/articles/s41....
Do other fusions also suppress EGFR? Also EML4-ALK formed condensates -- are condensates important?
Led by superb PhD student Carol Gao.
www.biorxiv.org/content/10.1...
Implications for drug tolerance/resistance, and includes one big surprise🫧.👇
Led by superb PhD student Carol Gao.
www.biorxiv.org/content/10.1...
Implications for drug tolerance/resistance, and includes one big surprise🫧.👇

Note that this session does *not* appear in the app for some reason

Note that this session does *not* appear in the app for some reason
registration: events.cancer.gov/nci/syntheti...
#SynSysBio4SpatialCancerResearch

registration: events.cancer.gov/nci/syntheti...
#SynSysBio4SpatialCancerResearch
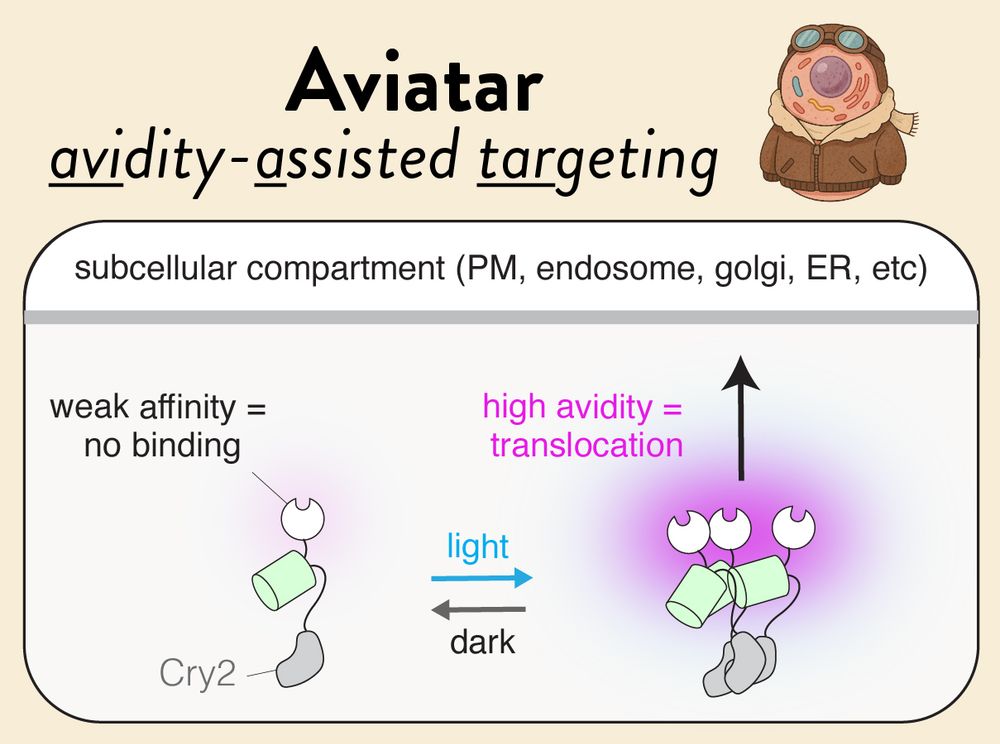
📝 tinyurl.com/aviatar
📝 tinyurl.com/aviatar
www.biorxiv.org/content/10.1...
Check out the 🧵 from first author Dennis Huang:
www.biorxiv.org/content/10.1...
Check out the 🧵 from first author Dennis Huang:
www.sciencedirect.com/science/arti...
www.sciencedirect.com/science/arti...

