Alejandro Cadranel
@cadralab.bsky.social
88 followers
130 following
7 posts
Chemistry · Physical · Inorganic || Artificial Photosynthesis · Transition Metal Complexes · Carbon Dots || Group Webpage: cadralab.framer.website
Posts
Media
Videos
Starter Packs
Reposted by Alejandro Cadranel
Reposted by Alejandro Cadranel
Oliver Wenger
@wengeroliver.bsky.social
· Jul 18
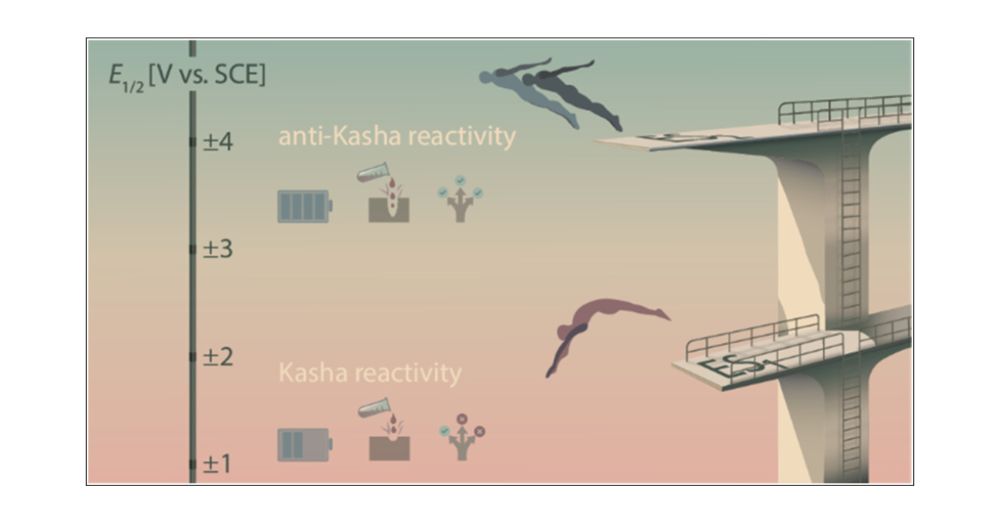
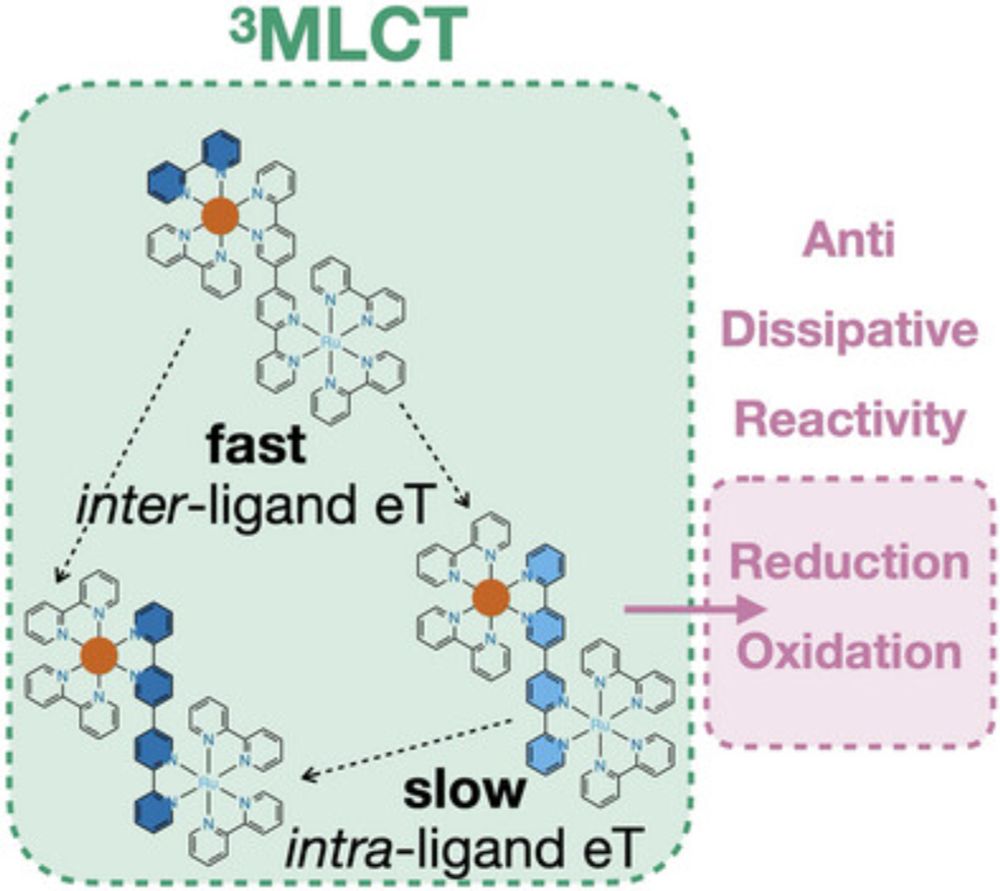
Breaking Kasha’s Rule to Enable Higher Reactivity in Photoredox Catalysis
Nearly all photochemical transformations known to date follow Kasha’s rule, implying that reactions occur only from the lowest electronically excited state of a given spin multiplicity due to the fast...
bit.ly
Reposted by Alejandro Cadranel
Ksenia Glusac
@ksenia-glusac.bsky.social
· Jun 24
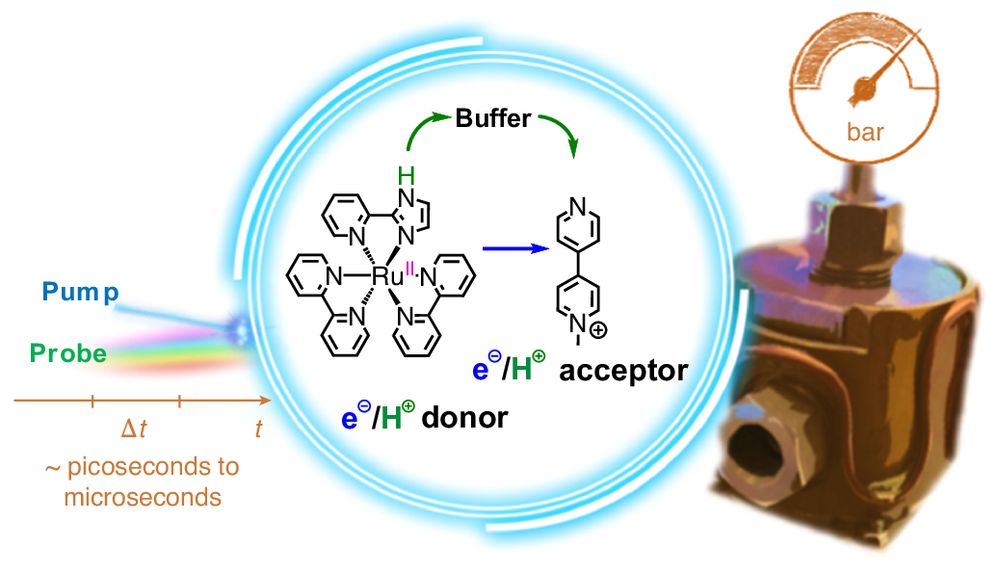
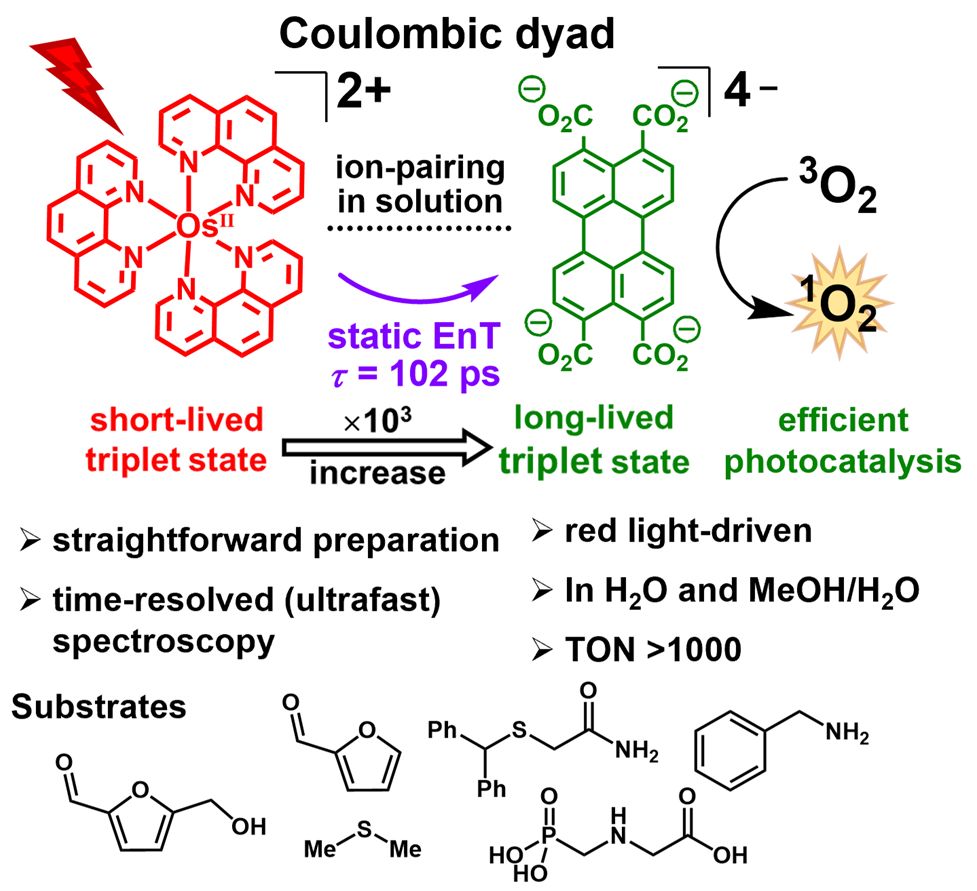
High-pressure pump–probe experiments reveal the mechanism of excited-state proton-coupled electron transfer and a shift from stepwise to concerted pathways
Nature Chemistry - Chemical energy conversion and storage rely on the selective movement of protons and electrons, thus understanding these processes is important for applications. Now experiments...
rdcu.be
Reposted by Alejandro Cadranel
Reposted by Alejandro Cadranel
Reposted by Alejandro Cadranel
Reposted by Alejandro Cadranel
Oliver Wenger
@wengeroliver.bsky.social
· Mar 27
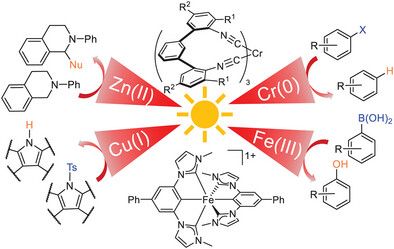
Molecular Design Principles for Photoactive Transition Metal Complexes: A Guide for “Photo-Motivated” Chemists
Luminescence and photochemistry involve electronically excited states that are inherently unstable and therefore spontaneously decay to electronic ground states, in most cases by nonradiative energy release that generates heat. This energy dissipation can occur on a time scale of 100 fs (∼10–13 s) and usually needs to be slowed down to at least the nanosecond (∼10–9 s) time scale for luminescence and intermolecular photochemistry to occur. This is a challenging task with many different factors to consider. An alternative emerging strategy is to target dissociative excited states that lead to metal–ligand bond homolysis on the subnanosecond time scale to access synthetically useful radicals. Based on a thorough review at the most recent advances in the field, this article aims to provide a concise guide to obtaining luminescent and photochemically useful coordination compounds with d-block elements. We hope to encourage “photo-motivated” chemists who have been reluctant to apply their synthetic and other knowledge to photophysics and photochemistry, and we intend to stimulate new approaches to the synthetic control of excited state behavior.
pubs.acs.org
Reposted by Alejandro Cadranel
Reposted by Alejandro Cadranel
Oliver Wenger
@wengeroliver.bsky.social
· Feb 28
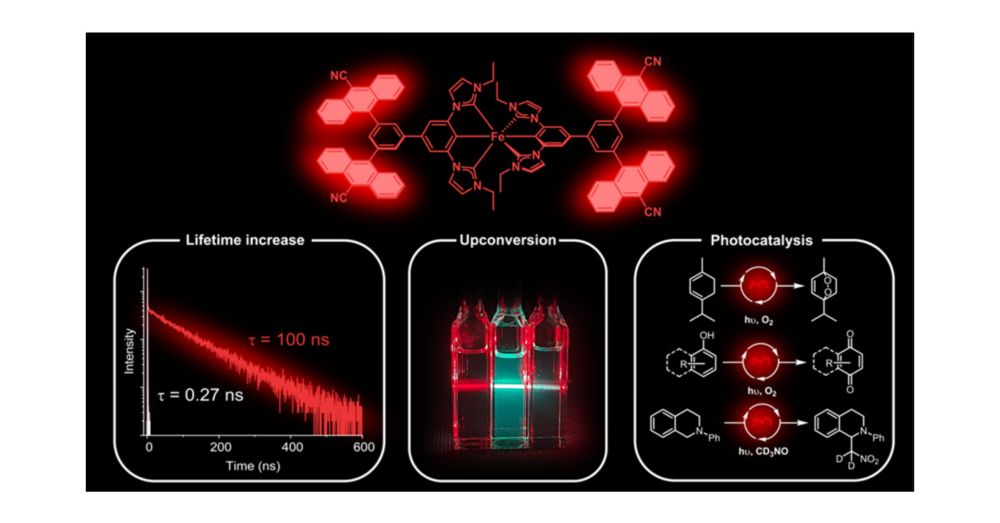
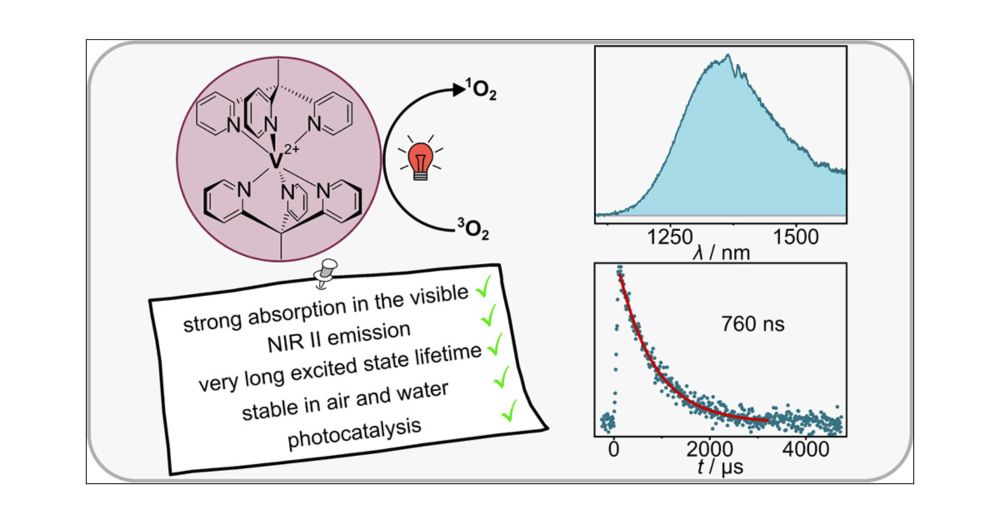
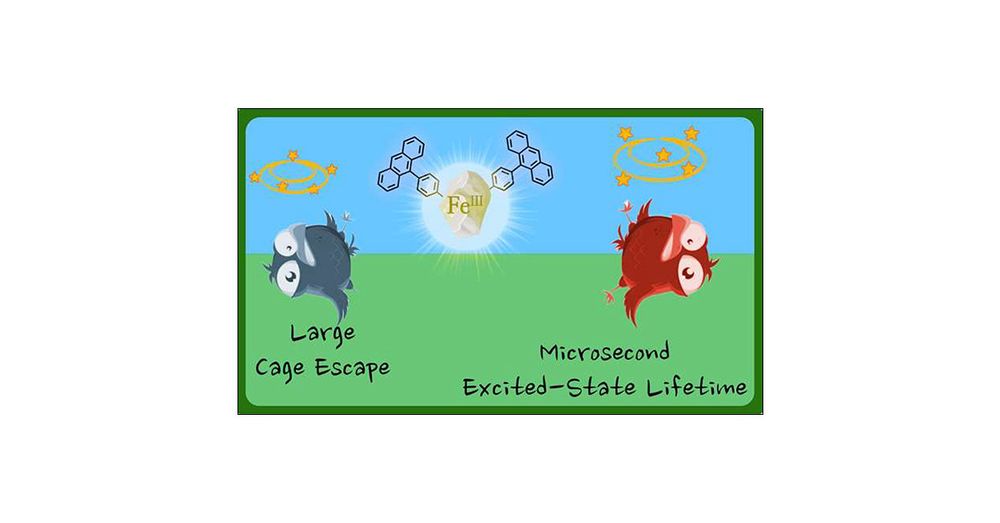
Iron(III) Complexes with Luminescence Lifetimes of up to 100 ns to Enhance Upconversion and Photocatalysis
Iron is the most abundant transition metal element and would be the ideal replacement for noble metals in many applications that rely on luminescent and long-lived electronically excited states. We sh...
pubs.acs.org
Alejandro Cadranel
@cadralab.bsky.social
· Feb 24
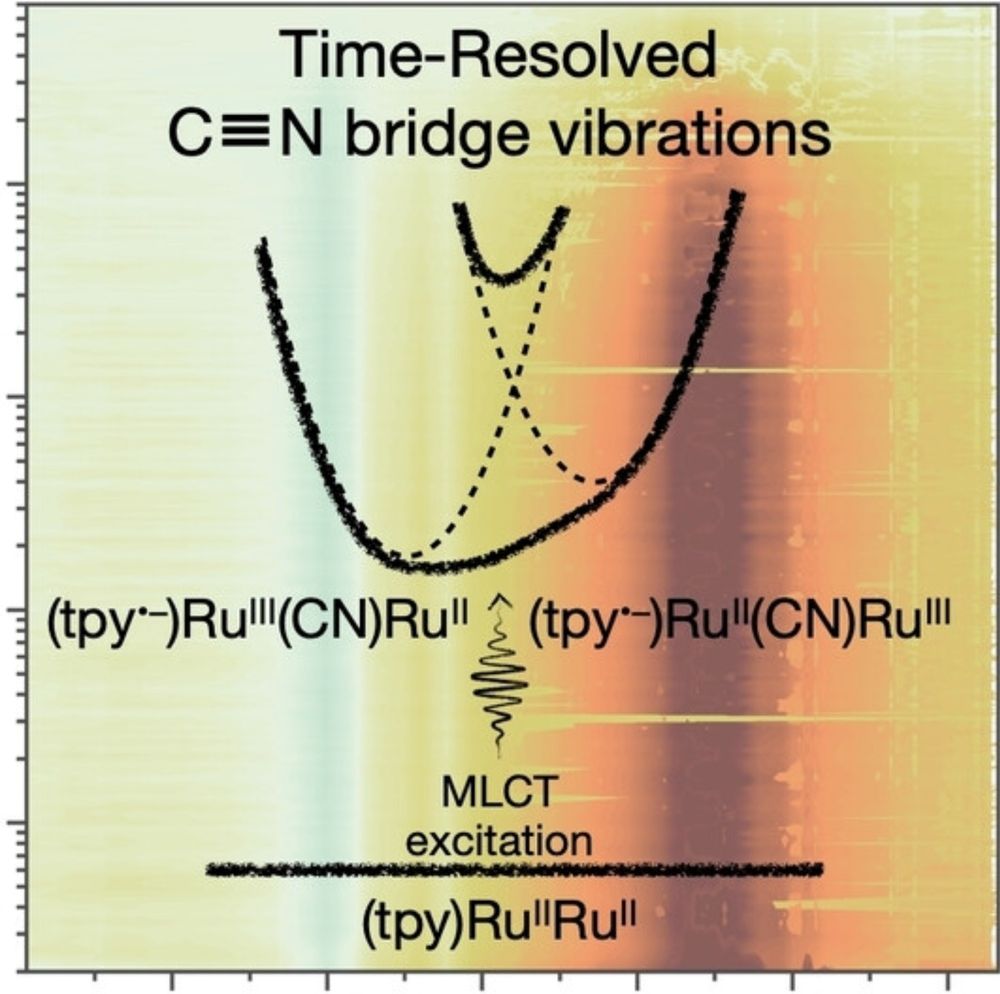
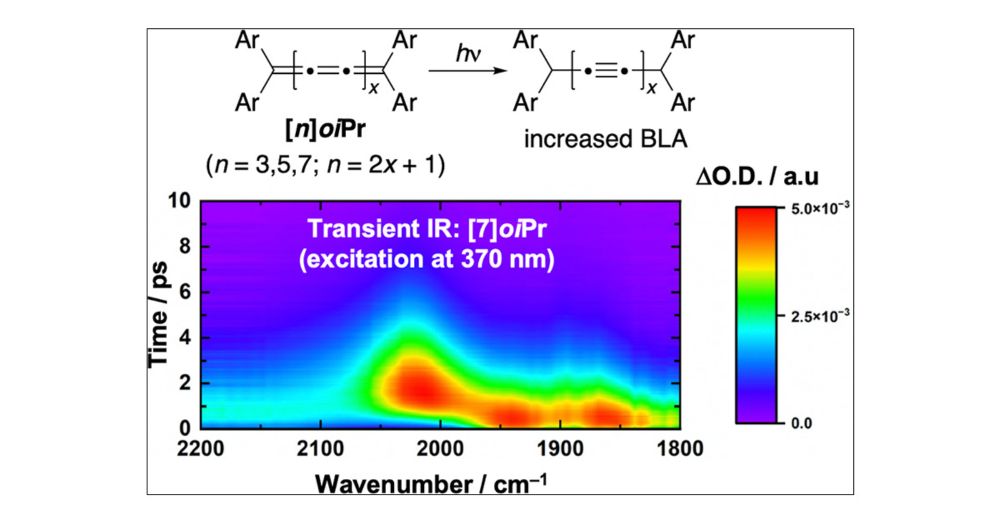
Barrierless Electron Transfer in a Photosynthetic Reaction Center Model
Ultrafast IR absorption spectroscopy was utilized to interrogate excited-state electron transfer in a strongly-coupled, cyanide-bridged photoinduced mixed-valent system. Despite structural asymmetry,...
onlinelibrary.wiley.com
Reposted by Alejandro Cadranel