Oliver Wenger
@wengeroliver.bsky.social
1.5K followers
430 following
13 posts
Professor of Chemistry at University of Basel, Switzerland
Posts
Media
Videos
Starter Packs
Reposted by Oliver Wenger
Reposted by Oliver Wenger
Reposted by Oliver Wenger
Reposted by Oliver Wenger
Oliver Wenger
@wengeroliver.bsky.social
· Aug 27
Oliver Wenger
@wengeroliver.bsky.social
· Aug 27

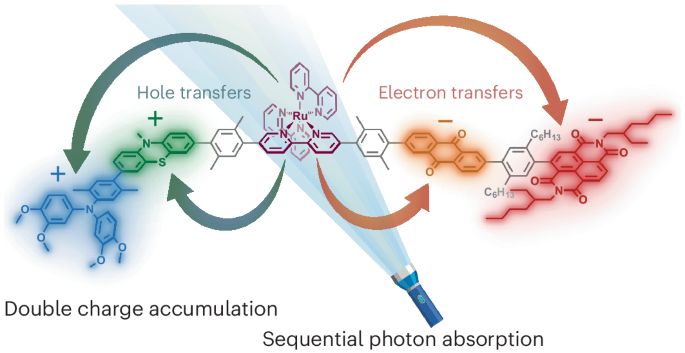
Photoinduced double charge accumulation in a molecular compound - Nature Chemistry
The photoinduced accumulation of redox equivalents is a challenging requirement for artificial photosynthesis. Now a molecule has been developed in which the sequential absorption of photons results i...
www.nature.com
Reposted by Oliver Wenger
University of Basel
@unibas.ch
· Aug 25

Chemists develop molecule for important step toward artificial photosynthesis
A research team from the University of Basel has developed a new molecule modeled on plant photosynthesis: under the influence of light, it stores two positive and two negative charges at the same tim...
www.unibas.ch
Reposted by Oliver Wenger
Reposted by Oliver Wenger
Reposted by Oliver Wenger
Malte Sellin
@msellin.bsky.social
· Jul 31

Revisiting CNC6F5: The Quest for Isocyanide Ligands with Strong π-Acceptor Properties Evaluated by Energy Decomposition Analysis
While perfluorinated isocyanide ligands such as CNCF3 and CNC6F5 have been known for decades, their use by organometallic chemists has been limited primarily due to the challenges associated with their cumbersome synthesis. In this study, we present an improved synthetic route to [Cr(CO)5(CNC6F5)] and present its structural characterization. For a set of isocyanide ligands (CNC6H5, p-CNC6H4F, CNCH3) and their perfluorinated counterparts (CNC6F5, CNCF3), Gibbs energies of complexation have been calculated with regard to a series of isoelectronic metal fragments [V(CO)5]−, [Cr(CO)5], [Mn(CO)5]+, and [Fe(CO)5]2+. Furthermore, the σ-donor and π-acceptor properties of these isocyanide ligands in the resulting complexes were analyzed using the EDA-NOCV method. For completeness, we have also included ligands such as CO, CNH, and N2 into the analysis. While only minor differences in complexation energies are observed for the Cr(CO)5 fragment, more pronounced effects have been observed for the charged complexes. Interestingly, perfluorinated isocyanide ligands show in all cases higher complexation energies than the carbonyl ligands, indicating their strong binding to metal centers. Their pronounced σ-donor and π-acceptor abilities reveal their potential suitability to stabilize metal centers in both positive and negative oxidation states.
pubs.acs.org
Reposted by Oliver Wenger
Oliver Wenger
@wengeroliver.bsky.social
· Jul 31
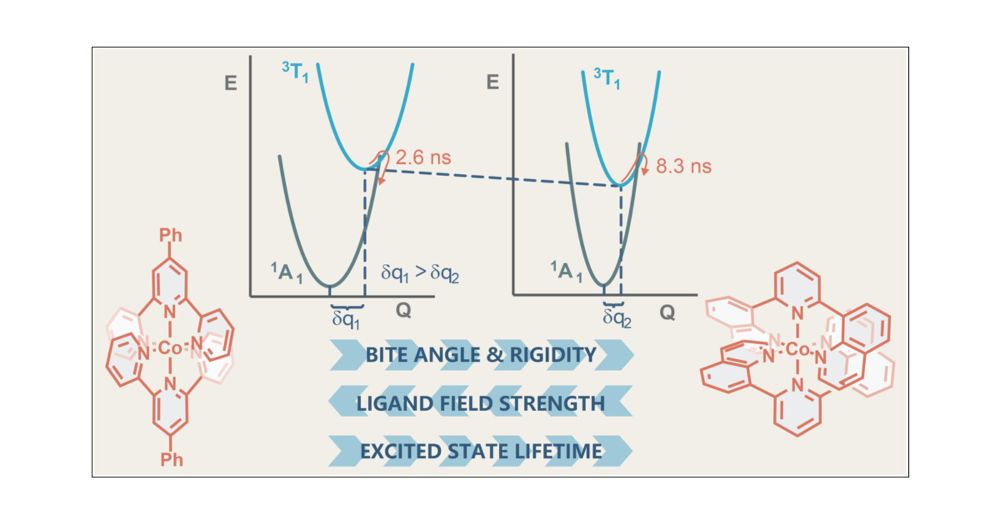
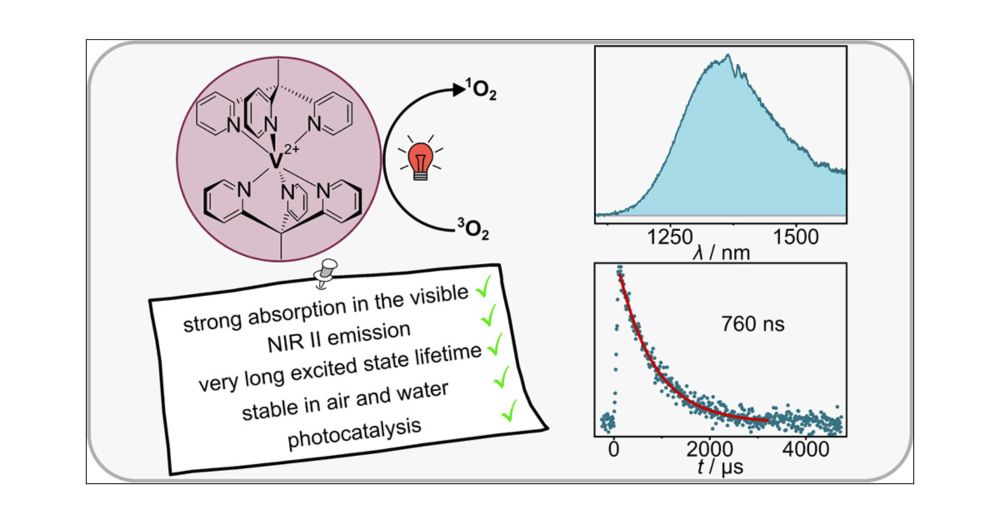
Structural Control of Metal-Centered Excited States in Cobalt(III) Complexes via Bite Angle and π–π Interactions
CoIII complexes have recently become an important focus in photophysics and photoredox catalysis due to metal-centered excited states with strong oxidizing properties. Optimizing chelate ligand bite a...
pubs.acs.org
Oliver Wenger
@wengeroliver.bsky.social
· Jul 29
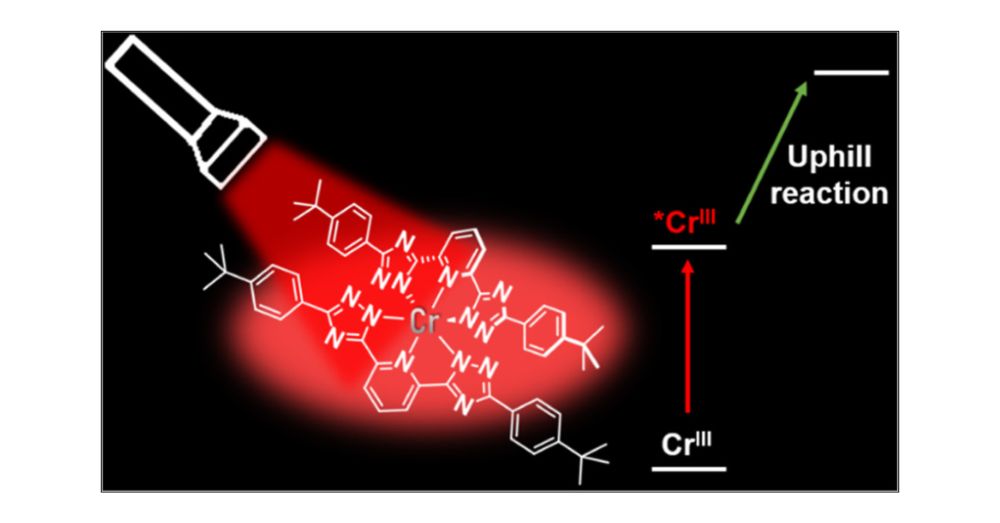
Pushing the Thermodynamic and Kinetic Limits of Near-Infrared Emissive CrIII Complexes in Photocatalysis
Photoactive CrIII complexes are typically based on polypyridine coordination environments, exhibit red luminescence, and are good photo-oxidants but have modest photoreducing properties. We report new...
pubs.acs.org
Oliver Wenger
@wengeroliver.bsky.social
· Jul 18
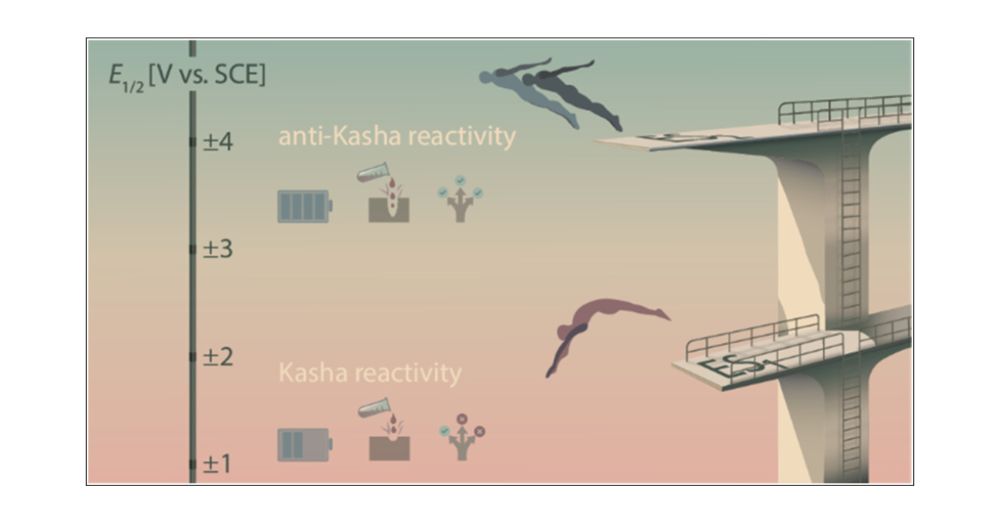
Breaking Kasha’s Rule to Enable Higher Reactivity in Photoredox Catalysis
Nearly all photochemical transformations known to date follow Kasha’s rule, implying that reactions occur only from the lowest electronically excited state of a given spin multiplicity due to the fast...
bit.ly
Reposted by Oliver Wenger
Reposted by Oliver Wenger
Reposted by Oliver Wenger
Bryan Kudisch
@bkudisch.bsky.social
· Jun 5

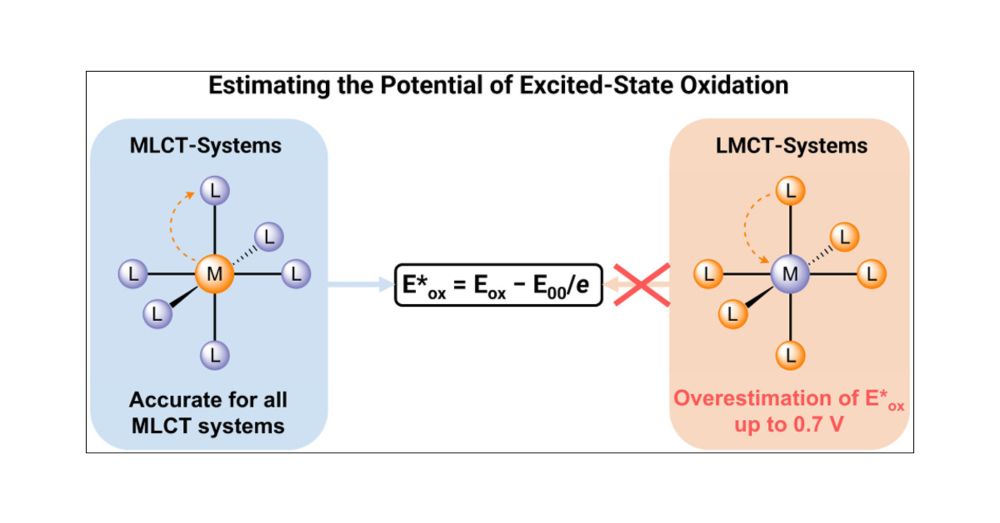
Ultrafast nonradiative relaxation limits the efficiency of photoinduced bond homolysis in molecular LMCT photocatalysts
Ligand-to-metal charge transfer photocatalysts (LMCT PCs) are being increasingly implemented towards construction and functionalization of organic molecules. Leveraging photoinduced metal-ligand bond ...
chemrxiv.org
Reposted by Oliver Wenger
Oliver Wenger
@wengeroliver.bsky.social
· Mar 27
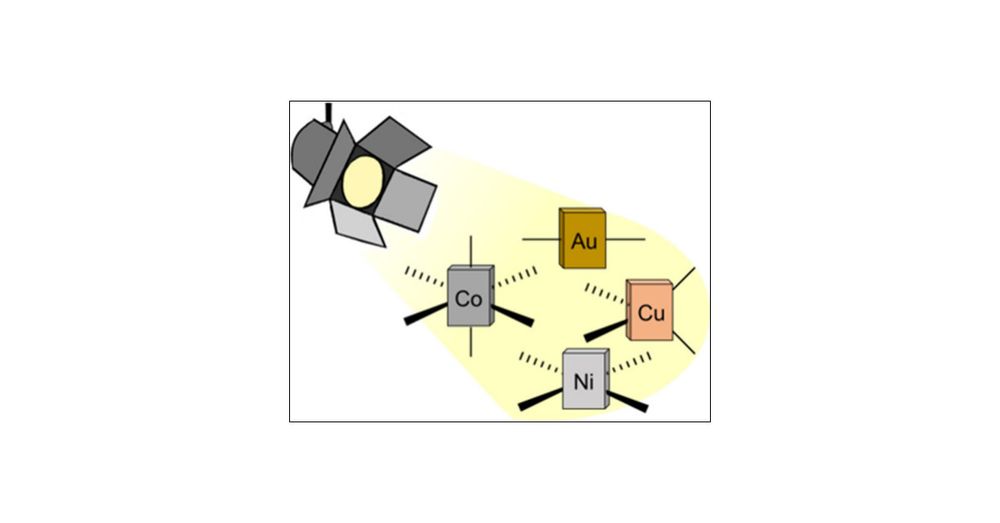
Molecular Design Principles for Photoactive Transition Metal Complexes: A Guide for “Photo-Motivated” Chemists
Luminescence and photochemistry involve electronically excited states that are inherently unstable and therefore spontaneously decay to electronic ground states, in most cases by nonradiative energy release that generates heat. This energy dissipation can occur on a time scale of 100 fs (∼10–13 s) and usually needs to be slowed down to at least the nanosecond (∼10–9 s) time scale for luminescence and intermolecular photochemistry to occur. This is a challenging task with many different factors to consider. An alternative emerging strategy is to target dissociative excited states that lead to metal–ligand bond homolysis on the subnanosecond time scale to access synthetically useful radicals. Based on a thorough review at the most recent advances in the field, this article aims to provide a concise guide to obtaining luminescent and photochemically useful coordination compounds with d-block elements. We hope to encourage “photo-motivated” chemists who have been reluctant to apply their synthetic and other knowledge to photophysics and photochemistry, and we intend to stimulate new approaches to the synthetic control of excited state behavior.
pubs.acs.org
Reposted by Oliver Wenger
Oliver Wenger
@wengeroliver.bsky.social
· Mar 27

Molecular Design Principles for Photoactive Transition Metal Complexes: A Guide for “Photo-Motivated” Chemists
Luminescence and photochemistry involve electronically excited states that are inherently unstable and therefore spontaneously decay to electronic ground states, in most cases by nonradiative energy release that generates heat. This energy dissipation can occur on a time scale of 100 fs (∼10–13 s) and usually needs to be slowed down to at least the nanosecond (∼10–9 s) time scale for luminescence and intermolecular photochemistry to occur. This is a challenging task with many different factors to consider. An alternative emerging strategy is to target dissociative excited states that lead to metal–ligand bond homolysis on the subnanosecond time scale to access synthetically useful radicals. Based on a thorough review at the most recent advances in the field, this article aims to provide a concise guide to obtaining luminescent and photochemically useful coordination compounds with d-block elements. We hope to encourage “photo-motivated” chemists who have been reluctant to apply their synthetic and other knowledge to photophysics and photochemistry, and we intend to stimulate new approaches to the synthetic control of excited state behavior.
pubs.acs.org