Craig Day
@craigday.bsky.social
800 followers
500 following
48 posts
Assistant Professor at the University of Copenhagen 🇩🇰🇨🇦 - Organometallics - Mechanism - Sustainability - Polymers
Posts
Media
Videos
Starter Packs
Reposted by Craig Day
Reposted by Craig Day
Jan H. Jensen
@janhjensen.bsky.social
· Mar 26
Reposted by Craig Day
Reposted by Craig Day
Reposted by Craig Day
Jan H. Jensen
@janhjensen.bsky.social
· Feb 18
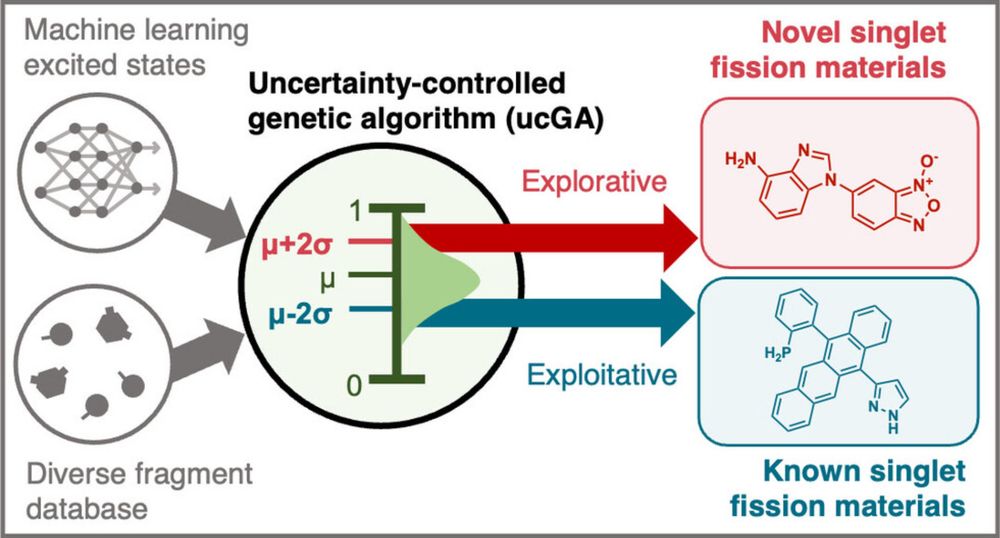
Inverse Design of Singlet‐Fission Materials with Uncertainty‐Controlled Genetic Optimization
We uncover candidates for singlet fission using a machine learning-driven uncertainty-controlled genetic algorithm (ucGA), which is run either in an exploitative or explorative manner. Leveraging a f...
doi.org
Reposted by Craig Day
Per-Ola Norrby
@peonor.bsky.social
· Feb 17
Chemistry Data Summer Internship
About AstraZeneca AstraZeneca is a global, science-led biopharmaceutical business and its innovative medicines are used by millions of patients worldwide. AstraZeneca has long been an advocate of stud...
astrazeneca.wd3.myworkdayjobs.com
Reposted by Craig Day
Reposted by Craig Day
Craig Day
@craigday.bsky.social
· Jan 28
Reposted by Craig Day
Jan H. Jensen
@janhjensen.bsky.social
· Jan 19
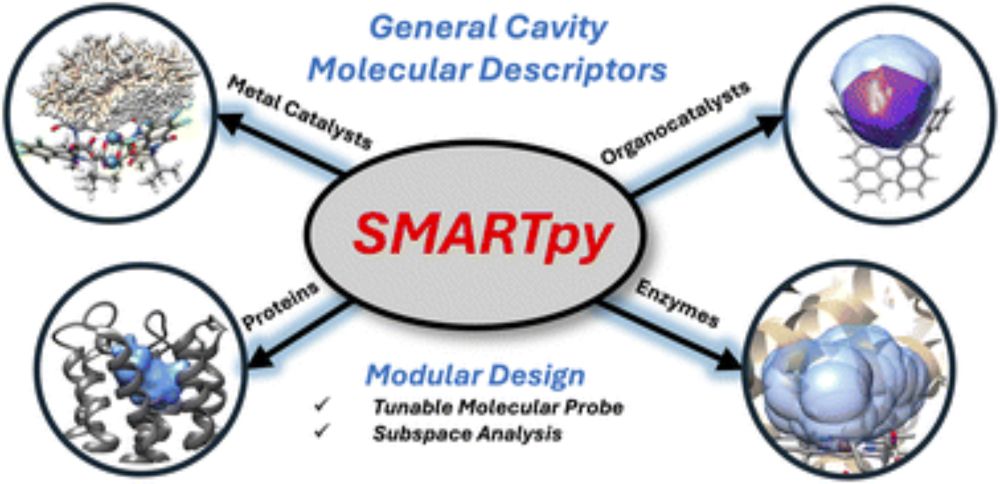
SMARTpy: a Python package for the generation of cavity steric molecular descriptors and applications to diverse systems
Steric molecular descriptors designed for machine learning (ML) applications are critical for connecting structure–function relationships to mechanistic insight. However, many of these descriptors are...
doi.org
Reposted by Craig Day
Reposted by Craig Day
Weix Group
@weixgroup.bsky.social
· Jan 13
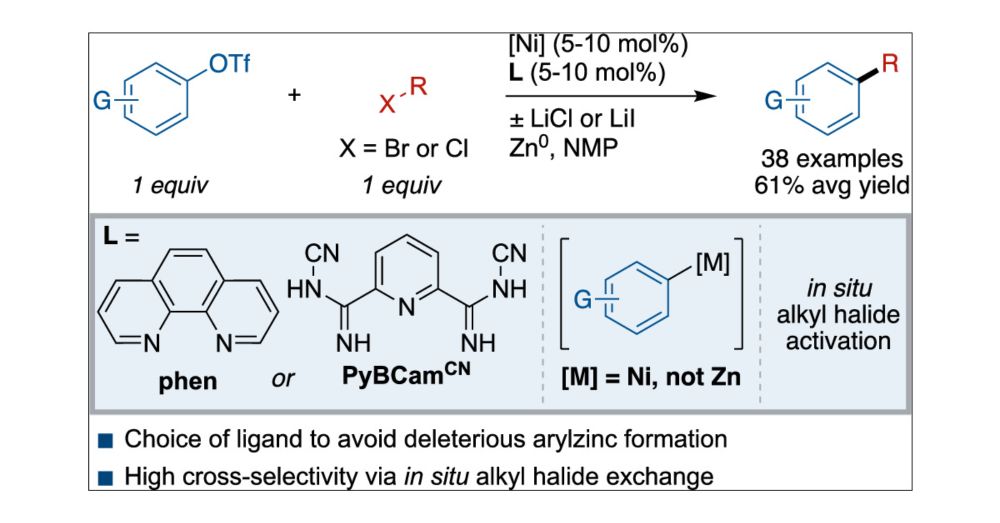
Nickel-Catalyzed Cross-Electrophile Coupling of Aryl Triflates with Alkyl Halides: Mechanism-Informed Design of More General Conditions
Aryl triflates make up a class of aryl electrophiles that are available in a single step from the corresponding phenol. Despite the known reactivity of nickel complexes for aryl C–O bond activation of phenol derivatives, nickel-catalyzed cross-electrophile coupling using aryl triflates has proven challenging. Herein, we report a method to form C(sp2)–C(sp3) bonds by coupling aryl triflates with alkyl bromides and chlorides using phenanthroline (phen) or pyridine-2,6-bis(N-cyanocarboxamidine) (PyBCamCN)-ligated nickel catalysts. The scope of the reaction is demonstrated with 38 examples (61 ± 14% average yield). Mechanistic studies provide a rationale for the conditions used and a roadmap for further applications of cross-electrophile coupling. First, the rate of alkyl radical generation is controlled by maintaining the majority of alkyl halide as the alkyl chloride, which is unreactive, and utilizing a dynamic halide exchange process to adjust the concentration of reactive alkyl bromide or iodide. Second, the challenge of using electron-rich aryl triflates appears to be due to off-cycle transmetalation to form unproductive aryl zinc reagents. The optimal PyBCamCN ligand together with LiCl avoids this deleterious transmetalation step.
doi.org
Craig Day
@craigday.bsky.social
· Jan 13
Craig Day
@craigday.bsky.social
· Jan 13
Craig Day
@craigday.bsky.social
· Jan 13
Craig Day
@craigday.bsky.social
· Jan 13
Reposted by Craig Day
Waser Group
@lcsolab.bsky.social
· Jan 11
Reposted by Craig Day
Reposted by Craig Day
Craig Day
@craigday.bsky.social
· Dec 27
Craig Day
@craigday.bsky.social
· Dec 6
Craig Day
@craigday.bsky.social
· Dec 6