Per-Ola Norrby
@peonor.bsky.social
2.9K followers
2K following
140 posts
Chemist, husband, father, singer. Sustainability, catalysis, stereochemistry, predictive modeling. Senior Principal Scientist @astrazeneca.bsky.social Gothenburg. he/him
Posts
Media
Videos
Starter Packs
Reposted by Per-Ola Norrby
Per-Ola Norrby
@peonor.bsky.social
· Aug 22
Precedent Finder – Locating Pareto-Optimal
Reactions
We present Precedent Finder, a cheminformatics search tool for locating relevant reaction
information in chemical reaction databases. Precedent Finder is a multiobjective optimization, in
that it re...
doi.org
Per-Ola Norrby
@peonor.bsky.social
· Aug 22

Predicting Reaction Feasibility and Selectivity of Aromatic C–H Thianthrenation with a QM-ML Hybrid Approach
The direct thianthrenation of aromatic C–H bonds is a valuable late-stage functionalization strategy that can assist, for example, the development of new drugs. We herein present a predictive computat...
doi.org
Per-Ola Norrby
@peonor.bsky.social
· Aug 19
Ruthenium-catalyzed synthesis of tricyclic 1,5-fused 1,2,3-triazole piperazines
A double cyclization strategy, involving sequential ruthenium-catalyzed azide alkyne cycloaddition (RuAAC) and hydrogen borrowing, allows the rapid assembly of tricyclic 1,5-fused 1,2,3-triazole piper...
doi.org
Reposted by Per-Ola Norrby
Robin Bedford
@bedcatalysis.bsky.social
· Jul 11
Per-Ola Norrby
@peonor.bsky.social
· Jun 17
Reposted by Per-Ola Norrby
Per-Ola Norrby
@peonor.bsky.social
· May 23
Reposted by Per-Ola Norrby
Lukas Sigmund
@lukasmsigmund.bsky.social
· May 19

Predicting Reaction Feasibility and Selectivity of Aromatic C–H Thianthrenation with a QM-ML Hybrid Approach
The direct thianthrenation of aromatic C–H bonds is a valuable late-stage functionalization strategy that can assist, for example, the development of new drugs. We herein present a predictive computat...
chemrxiv.org
Per-Ola Norrby
@peonor.bsky.social
· Apr 22
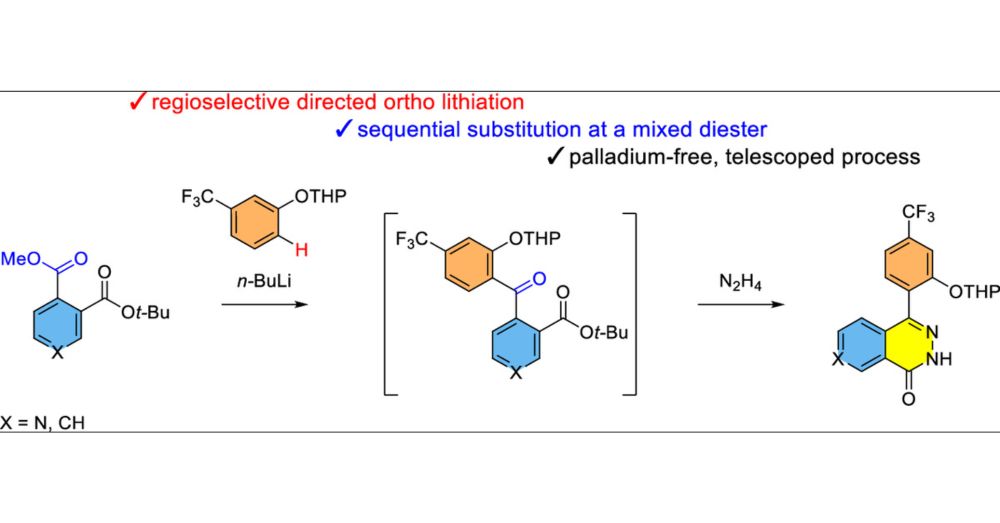
Access to Phenolic Pyridopyridazinones and Phthalazinones Using THP Ether-Directed Ortho Lithiation
Route scouting, process research and development, and large-scale synthesis of phenol-substituted pyridopyridazinones (azaphthalazinones) and phthalazinones are reported. For the introduction of one o...
doi.org
Per-Ola Norrby
@peonor.bsky.social
· Mar 13
Per-Ola Norrby
@peonor.bsky.social
· Mar 10
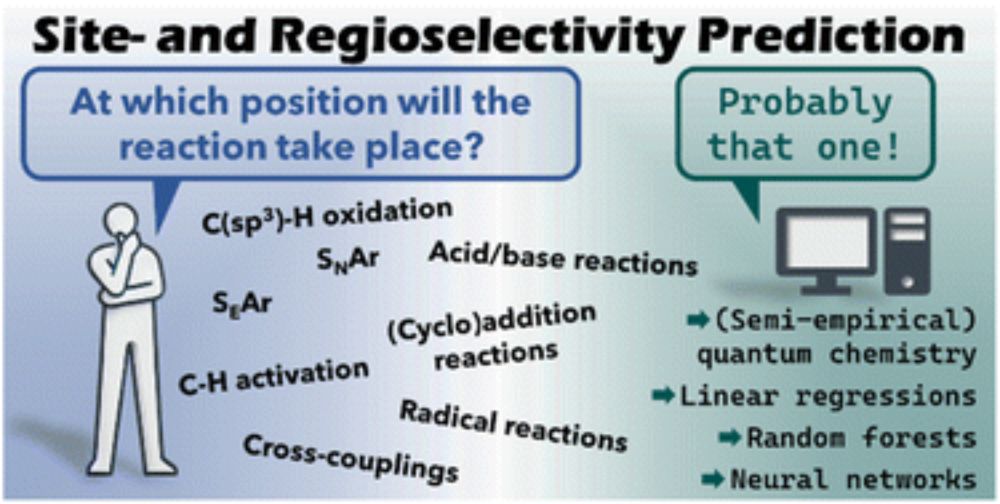
Computational tools for the prediction of site- and regioselectivity of organic reactions
The regio- and site-selectivity of organic reactions is one of the most important aspects when it comes to synthesis planning. Due to that, massive research efforts were invested into computational mo...
doi.org
Reposted by Per-Ola Norrby
Per-Ola Norrby
@peonor.bsky.social
· Feb 26
Per-Ola Norrby
@peonor.bsky.social
· Feb 26
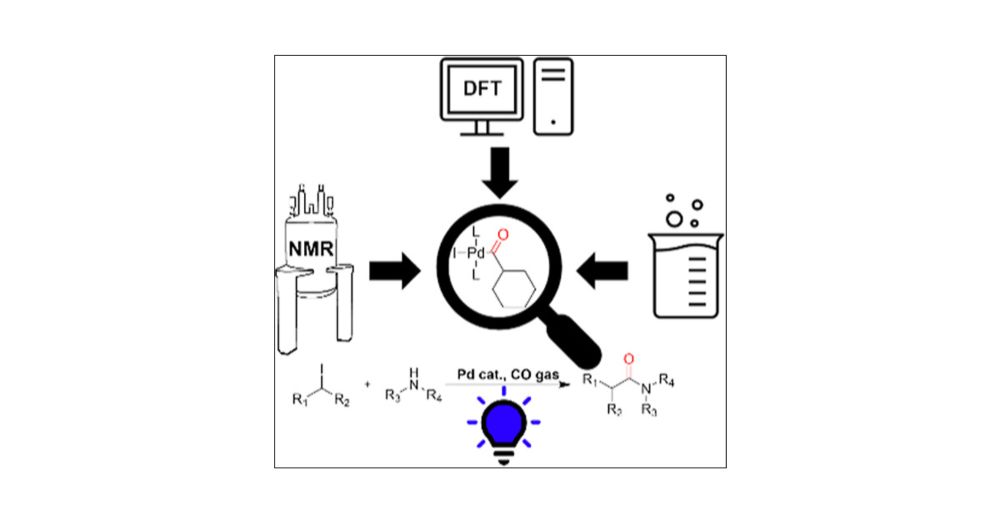
Mechanistic Study of Photochemical Aminocarbonylation of Alkyl Iodides Catalyzed by a Palladium Catalyst Using Experimental and Computational Methods
Aminocarbonylations are versatile reactions amenable to applications in convergent synthesis and isotope labeling. Herein, a mechanistic study of a previously reported visible-light-promoted aminocarbonylation of unactivated alkyl iodides is presented. This study combines in situ spectroscopy, computational chemistry, and organic chemistry techniques. A T1 excited-state promoted ligand dissociation in concert with an atom transfer radical addition was uncovered as a likely first step in the mechanism, instead of the usual three-center oxidative addition. Improvement in the reaction yield was achieved by optimizing the reaction based on mechanistic insights. This took the form of promoting a computationally uncovered cationic carbonylation pathway with the use of bidentate ligands.
doi.org
Per-Ola Norrby
@peonor.bsky.social
· Feb 17





